| 1 |
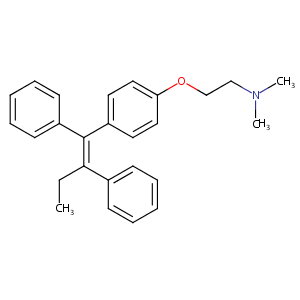
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1016).
|
| 3 |
Tamoxifen FDA Label
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 5 |
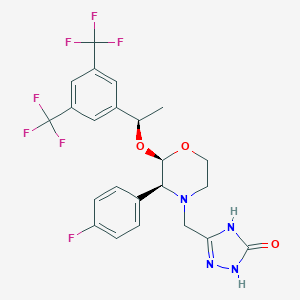
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3490).
|
| 6 |
Aprepitant FDA Label
|
| 7 |
ClinicalTrials.gov (NCT00571168) Efficacy and Safety of Aprepitant in Subjects With Multiple Myeloma During and After High-dose Chemotherapy. U.S. National Institutes of Health.
|
| 8 |
Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause. Recent Pat Cardiovasc Drug Discov. 2008 Nov;3(3):165-86.
|
| 9 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 10 |
The phytoestrogen genistein enhances multidrug resistance in breast cancer cell lines by translational regulation of ABC transporters. Cancer Lett. 2016 Jun 28;376(1):165-72.
|
| 11 |
Modulation of the ATPase and transport activities of broad-acting multidrug resistance factor ABCC10 (MRP7). Cancer Res. 2012 Dec 15;72(24):6457-67.
|
| 12 |
Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004 Sep;310(3):1062-75.
|
| 13 |
Endoxifen and other metabolites of tamoxifen inhibit human hydroxysteroid sulfotransferase 2A1 (hSULT2A1). Drug Metab Dispos. 2014 Nov;42(11):1843-50.
|
| 14 |
Functional significance of UDP-glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res. 2009 Mar 1;69(5):1892-900.
|
| 15 |
Genotoxicity of tamoxifen, tamoxifen epoxide and toremifene in human lymphoblastoid cells containing human cytochrome P450s. Carcinogenesis. 1994 Jan;15(1):5-9.
|
| 16 |
Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4'-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos. 2002 Aug;30(8):869-74.
|
| 17 |
Tamoxifen-induced adduct formation and cell stress in human endometrial glands. Drug Metab Dispos. 2010 Jan;38(1):200-7.
|
| 18 |
CYP2D6 polymorphisms and tamoxifen metabolism: clinical relevance. Curr Oncol Rep. 2010 Jan;12(1):7-15.
|
| 19 |
Polymorphisms in cytochrome P4503A5 (CYP3A5) may be associated with race and tumor characteristics, but not metabolism and side effects of tamoxifen in breast cancer patients. Cancer Lett. 2005 Jan 10;217(1):61-72.
|
| 20 |
Polymorphism of estrogen metabolism genes and cataract. Med Hypotheses. 2004;63(3):494-7.
|
| 21 |
Drug Interactions Flockhart Table
|
| 22 |
Tamoxifen inhibits cytochrome P450 2C9 activity in breast cancer patients. J Chemother. 2006 Aug;18(4):421-4.
|
| 23 |
Effect of tamoxifen on the enzymatic activity of human cytochrome CYP2B6. J Pharmacol Exp Ther. 2002 Jun;301(3):945-52.
|
| 24 |
Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006 Mar 13;25(11):1679-91.
|
| 25 |
Metabolism and transport of tamoxifen in relation to its effectiveness: new perspectives on an ongoing controversy. Future Oncol. 2014 Jan;10(1):107-22.
|
| 26 |
Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab Dispos. 2007 Nov;35(11):2006-14.
|
| 27 |
Factor V Leiden mutation and thromboembolism risk in women receiving adjuvant tamoxifen for breast cancer. J Natl Cancer Inst. 2010 Jul 7;102(13):942-9. doi: 10.1093/jnci/djq211. Epub 2010 Jun 16.
|
| 28 |
Potent, brain-penetrant, hydroisoindoline-based human neurokinin-1 receptor antagonists. J Med Chem. 2009 May 14;52(9):3039-46.
|
| 29 |
Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109.
|
| 30 |
Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patients. Cancer Chemother Pharmacol. 2005 Jun;55(6):609-16.
|
| 31 |
Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos. 2004 Nov;32(11):1287-92.
|
| 32 |
Neurokinin-1 receptor (NK1R) inhibition sensitizes APL cells to anti-tumor effect of arsenic trioxide via restriction of NF-B axis: Shedding new light on resistance to Aprepitant. Int J Biochem Cell Biol. 2018 Oct;103:105-114. doi: 10.1016/j.biocel.2018.08.010. Epub 2018 Aug 23.
|
|
|
|
|
|
|