Details of the Drug
General Information of Drug (ID: DMGFV3Z)
| Drug Name |
Nizatidine
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acinon; Antizid; Axid; Calmaxid; Cronizat; Distaxid; Galitidin; Gastrax; Naxidine; Niatidine; Nizatidina; Nizatidinum; Nizax; Nizaxid; Panaxid; Tazac; Ulcosol; Ulxid; Zanizal; Zinga; Axid Ar; Nizatidina [Spanish]; Nizatidinum [Latin]; Splendil ER; LY 139037; Acinon (TN); Axid (TN); LY-139037; Tazac (TN); ZE-101; ZL-101; Nizatidine (JAN/USP/INN); Nizatidine [USAN:BAN:INN:JAN]; N-(2-(((2-((Dimethylamino)methyl)-4-thiazolyl)methyl)thio)ethyl)-N'-methyl-2-nitro-1,1-ethenediamine; N-(4-(6-Methylamino-7-nitro-2-thia-5-aza-6-hepten-1-yl)-2-thiazolylmethyl)-N,N-dimethylamin; (E)-1-N'-[2-[[2-(dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine; (E)-N-[2-[[2-(dimethylaminomethyl)-1,3-thiazol-4-yl]methylsulfanyl]ethyl]-N'-methyl-2-nitroethene-1,1-diamine; (E)-N-{2-[({2-[(dimethylamino)methyl]-1,3-thiazol-4-yl}methyl)thio]ethyl}-N'-methyl-2-nitroethene-1,1-diamine
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiulcer Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
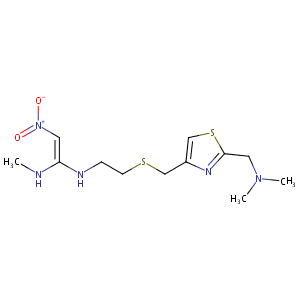 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 331.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Nizatidine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7248). | ||||
|---|---|---|---|---|---|
| 2 | Nizatidine FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Does the use of nizatidine, as a pro-kinetic agent, improve gastric emptying in patients post-oesophagectomy J Gastrointest Surg. 2009 Mar;13(3):432-7. | ||||
| 7 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | ||||
| 8 | DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB00585) | ||||
| 9 | Effects of histamine H(2)-receptor antagonists on human plasma levels of calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J Pharm Pharmacol. 2002 Nov;54(11):1559-63. doi: 10.1211/002235702117. | ||||
| 10 | Albin H, Vincon G, Begaud B, Bistue C, Perez P "Effect of aluminum phosphate on the bioavailability of ranitidine." Eur J Clin Pharmacol 32 (1987): 97-9. [PMID: 3582475] | ||||
| 11 | Honig PK, Gillespie BK "Clinical significance of pharmacokinetic drug interactions with over-the-counter (OTC) drugs." Clin Pharmacokinet 35 (1998): 167-71. [PMID: 9784931] | ||||
| 12 | Dey NG, Castleden CM, Ward J, et al "The effect of cimetidine on tolbutamide kinetics." Br J Clin Pharmacol 16 (1983): 438-40. [PMID: 6626438] | ||||
| 13 | Alffenaar JW, van Assen S, van der Werf TS, Kosterink JG, Uges DR "Omeprazole significantly reduces posaconazole serum trough level." Clin Infect Dis 48 (2009): 839. [PMID: 19220151] | ||||
| 14 | Anderson JR, Poklis A, Slavin RG "A fatal case of theophylline intoxication." Arch Intern Med 143 (1983): 559-60. [PMID: 6830388] | ||||
| 15 | Product Information. Spectracef (cefditoren). TAP Pharmaceuticals Inc, Deerfield, IL. | ||||
| 16 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 17 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 18 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 19 | Blum RA, D'Andrea DT, Florentino BM, et al "Increased gastric pH and the bioavailability of fluconazole and ketoconazole." Ann Intern Med 114 (1991): 755-7. [PMID: 2012358] | ||||
| 20 | Adachi M, Hinatsu Y, et.al "Improved dissolution and absorption of ketoconazole in the presence of organic acids as pH-modifiers." Eur J Pharm Sci 76 (2015): 225-30. [PMID: 25988287] | ||||
| 21 | Product Information. Harvoni (ledipasvir-sofosbuvir). Gilead Sciences, Foster City, CA. | ||||
| 22 | Product Information. Rescriptor (delavirdine). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 23 | Product Information. Lexiva (fosamprenavir). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 24 | Product Information. Edurant (rilpivirine). Tibotec Pharmaceuticals, Titusville, NJ. | ||||
| 25 | Product Information. Isentress (raltegravir). Merck & Company Inc, West Point, PA. | ||||
| 26 | Product Information. Zykadia (ceritinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 27 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 28 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 29 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 30 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 31 | Product Information. Effient (prasugrel). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 32 | Product Information. Inlyta (axitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
