Details of the Drug
General Information of Drug (ID: DMXNZM4)
| Drug Name |
Oxazepam
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Abboxampam; Abboxapam; Adumbran; Alepam; Ansioxacepam; Anxiolit; Aplakil; Astress; Azutranguil; Azutranquil; Bonare; Drimuel; Droxacepam; Durazepam; Hilong; Isodin; Lederpam; Limbial; Murelax; Nesontil; Noctazepam; Nortemazepam; Nozepam; Ossazepam; Oxanid; Oxazepamum; Oxazipam; Oxozepam; Pacienx; Praxiten; Propax; Psicopax; Psiquiwas; QUEN; Quilibrex; Rondar; Sedigoa; Serax; Serenal; Serenid; Serepax; Seresta; Serpax; Sigacalm; Sobril; Tacepam; Tarchomin; Tazepam; Uskan; Vaben; Zaxopam; Anxiolit retard; Ossazepam [DCIT]; P acienx; CB 8092; DF2371850; O5254_SIGMA; Wy 3498; Z 10 tr; Alepam (TN); Apo-astress; Hi-Long; Medopam (TN); Murelax (TN); N-Desmethyltemazepam; Noripam (TN); Oxa-puren; Oxazepamum [INN-Latin]; Purata (TN); Ro 5-6789; Serax (TN); Serenid-D; Serepax (TN); WY-3498; Wy-3498 stic; Z10-Tr; Ox-Pam (TN); Tranquo-buscopan-wirkstoff; Oxazepam (JAN/USP/INN); Oxazepam [USAN:INN:BAN:JAN]; (+-)-7-Chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepin-2-one; (+-)-Oxazepam; (RS)-Oxazepam; 1,3-Dihydro-7-chloro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepin-2-one; 1,3-Dihydro-7-chloro-3-hydroxy-5-phenyl-3H-1,4-benzodiazepin-2-one; 7-Chloro-1,3-dihydro-3-hydroxy-5-phenyl-1,4(2H)-benzodiazepin-2-one; 7-Chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepin-2-one; 7-Chloro-1,3-dihydro-3-hydroxy-5-phenyl-2H-1,4-benzodiazepine-2-one; 7-Chloro-3-hydroxy-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-1,4-benzodiazepin-2-one
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
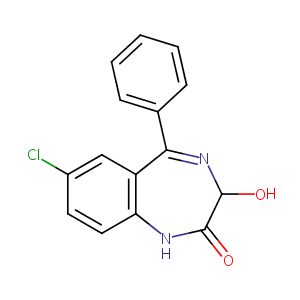 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 286.71 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Oxazepam (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Oxazepam FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7253). | ||||
| 3 | FDA Approved Drug Products: Oxazepam oral capsules | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Health Canada Product Monograph: Oxazepam oral tablets | ||||
| 8 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 9 | Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol Biochem Behav. 2008 Nov;91(1):181-9. | ||||
| 10 | Bioreactor systems in drug metabolism: synthesis of cytochrome P450-generated metabolites. Metab Eng. 2000 Apr;2(2):115-25. | ||||
| 11 | Evidence for oxazepam as an in vivo probe of UGT2B15: oxazepam clearance is reduced by UGT2B15 D85Y polymorphism but unaffected by UGT2B17 deletion. Br J Clin Pharmacol. 2009 Nov;68(5):721-30. | ||||
| 12 | Cytochrome P450 enzyme activity and protein expression in primary porcine enterocyte and hepatocyte cultures. Xenobiotica. 2000 Jan;30(1):27-46. | ||||
| 13 | Stereoselective conjugation of oxazepam by human UDP-glucuronosyltransferases (UGTs): S-oxazepam is glucuronidated by UGT2B15, while R-oxazepam is glucuronidated by UGT2B7 and UGT1A9. Drug Metab Dispos. 2002 Nov;30(11):1257-65. | ||||
| 14 | (S)oxazepam glucuronidation is inhibited by ketoprofen and other substrates of UGT2B7. Pharmacogenetics. 1995 Feb;5(1):43-9. doi: 10.1097/00008571-199502000-00005. | ||||
| 15 | Chun AH, Carrigan PJ, Hoffman DJ, Kershner RP, Stuart JD "Effect of antacids on absorption of clorazepate." Clin Pharmacol Ther 22 (1977): 329-35. [PMID: 19188] | ||||
| 16 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 17 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 18 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 19 | Backman JT, Olkkola KT, Ojala M, Laaksovirta H, Neuvonen PJ "Concentrations and effects of oral midazolam are greatly reduced in patients treated with carbamazepine or phenytoin." Epilepsia 37 (1996): 253-7. [PMID: 8598183] | ||||
| 20 | Abernethy DR, Greenblatt DJ, Ameer B, Shader RI "Probenecid impairment of acetaminophen and lorazepam clearance: direct inhibition of ether glucuronide formation." J Pharmacol Exp Ther 234 (1985): 345-9. [PMID: 4020675] | ||||
| 21 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 22 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 23 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 24 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 25 | Product Information. Gattex (teduglutide). NPS Pharmaceuticals, Bedminster, NJ. | ||||
| 26 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 27 | van Hecken AM, Tjandramaga TB, Verbesselt R, de Schepper PJ "The influence of diflunisal on the pharmacokinetics of oxazepam." Br J Clin Pharmacol 20 (1985): 225-34. [PMID: 4041343] | ||||
| 28 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 29 | Kerin NZ, Aragon E, Faitel K, Frumin H, Rubenfire M "Long-term efficacy and toxicity of high- and low-dose amiodarone regimens." J Clin Pharmacol 29 (1989): 418-23. [PMID: 2661600] | ||||
