Details of the Drug
General Information of Drug (ID: DMP9TWZ)
| Drug Name |
Corticotropin
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
corticotropin; ACTH; Cortrophin; Corticotrophin; Adrenocorticotropic hormone; Corticotrophine; Corticotrofina; Acthargel; Corticotrophinum; beta-Corticotropin; Adrenocorticotrophin; Purified Cortrophin gel; Corticotropin [USP:INN]; 9002-60-2; CHEBI:3892; BDBM82408; ACTH-(1-39); 25-Asp-30-Gln-corticotropin porcine; NCGC00167127-01; CAS_12279-41-3; Adrenocorticotropic Hormone (1-39), human; LS-187380; SYSMEHFRWGKPVGKKRRPVKVYPDGAEDQLAEAFPLEF; J-004856; alpha1-39-Corticotropin (swine), 25-L-aspartic acid-30-L-glutamine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Sequence |
SYSMEHFRWGKPVGKKRRPVKVYPDGAEDQLAEAFPLEF
|
||||||||||||||||||||||||||||||
| Structure |
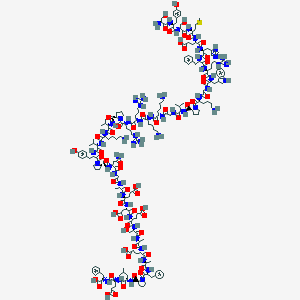 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 4541 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -19.7 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 148 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 63 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 68 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Corticotropin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
