Details of the Drug
General Information of Drug (ID: DMR5ZUP)
| Drug Name |
Paramethadione
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Isoethadione; Paradione; Parametadiona; Parametadione; Paramethadionum; Parametadione [DCIT]; A 348; Paradione (TN); Parametadiona [INN-Spanish]; Paramethadione (INN); Paramethadione [BAN:INN]; Paramethadione [INN:BAN]; Paramethadionum [INN-Latin]; 3,5-Dimethyl-5-ethyloxazolidine-2,4-dione; 5-Ethyl-3,5-dimethyl-1,3-oxazolidine-2,4-dione; 5-Ethyl-3,5-dimethyl-2,4-oxazolidinedione
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticonvulsants
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
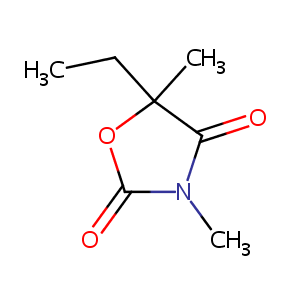 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 157.17 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Absence epilepsy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Paramethadione (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
