Details of the Drug
General Information of Drug (ID: DMYP4XC)
| Drug Name |
Norgestimate
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Norgestimato; Norgestimatum; Dexnorgestrel acetime; Norgestrel oxime acetate; D 138; ORF 10131; RWJ 10131; Norgestimato [INN-Spanish]; Norgestimatum [INN-Latin]; ORF-10131; Ortho-Prefest; RWJ-10131; Norgestimate (USP/INN); Norgestimate [USAN:INN:BAN]; D-13beta-Ethyl-17alpha-ethynyl-17beta-acetoxygon-4-en-3-oneoxime; D-13-beta-Ethyl-17-alpha-ethynyl-17-beta-acetoxygon-4-en-3-one oxime; [(3E,8R,9S,10R,13S,14S,17R)-13-ethyl-17-ethynyl-3-hydroxyimino-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl] acetate; (+)-13-Ethyl-17-hydroxy-18,19-dinor-17alpha-pregn-4-en-20-yn-3-one oxime acetate (ester); (17-alpha)-17-(Acetyloxy)-13-ethyl-18,19-dinorpregn-4-en-20-yn-3-one 3-oxime; (17alpha)-17-(Acetyloxy)-13-ethyl-18,19-dinorpregn-4-en-20-yn-3-one 3-oxime; (3E)-17alpha-ethynyl-3-(hydroxyimino)-18a-homoestr-4-en-17beta-yl acetate
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Contraceptive Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
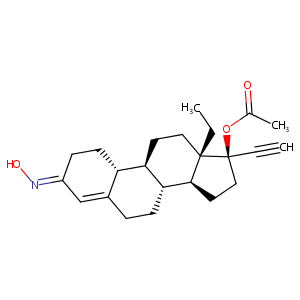 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 369.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.1 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acne vulgaris | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ED80 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Norgestimate (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
