| 1 |
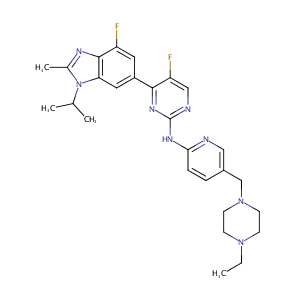
ClinicalTrials.gov (NCT02057133) A Study of LY2835219 (Abemaciclib) in Combination With Therapies for Breast Cancer That Has Spread
|
| 2 |
2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7382).
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7073).
|
| 5 |
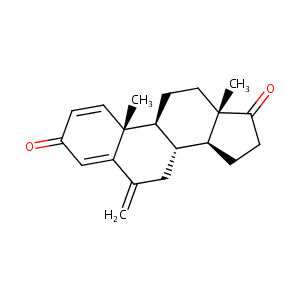
Exemestane FDA Label
|
| 6 |
Biological specificity of CDK4/6 inhibitors: dose response relationship, in vivo signaling, and composite response signature. Oncotarget. 2017 Jul 4;8(27):43678-43691. doi: 10.18632/oncotarget.18435.
|
| 7 |
Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41.
|
| 8 |
Abemaciclib Inhibits Renal Tubular Secretion Without Changing Glomerular Filtration Rate. Clin Pharmacol Ther. 2019 May;105(5):1187-1195.
|
| 9 |
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]-Abemaciclib.
|
| 10 |
CDK4/6 Inhibition Controls Proliferation of Bladder Cancer and Transcription of RB1. J Urol. 2016 Mar;195(3):771-9. doi: 10.1016/j.juro.2015.08.082. Epub 2015 Aug 28.
|
| 11 |
Effect of abemaciclib (LY2835219) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo. Biochem Pharmacol. 2017 Jan 15;124:29-42. doi: 10.1016/j.bcp.2016.10.015. Epub 2016 Nov 2.
|
| 12 |
In vitro therapeutic effects of abemaciclib on triple-negative breast cancer cells. J Biochem Mol Toxicol. 2021 Sep;35(9):e22858. doi: 10.1002/jbt.22858. Epub 2021 Jul 26.
|
| 13 |
Aromatase inhibitors--theoretical concept and present experiences in the treatment of endometriosis. Zentralbl Gynakol. 2003 Jul-Aug;125(7-8):247-51.
|
| 14 |
In vitro metabolism of exemestane by hepatic cytochrome P450s: impact of nonsynonymous polymorphisms on formation of the active metabolite 17beta-dihydroexemestane. Pharmacol Res Perspect. 2017 Apr 27;5(3):e00314.
|
| 15 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 16 |
Characterization of the weak estrogen receptor alpha agonistic activity of exemestane. Breast Cancer Res Treat. 2009 Aug;116(3):461-70. doi: 10.1007/s10549-008-0151-x. Epub 2008 Aug 3.
|
| 17 |
Effects of aromatase inhibitors on human osteoblast and osteoblast-like cells: a possible androgenic bone protective effects induced by exemestane. Bone. 2007 Apr;40(4):876-87. doi: 10.1016/j.bone.2006.11.029. Epub 2006 Dec 28.
|
| 18 |
Identifying environmental chemicals as agonists of the androgen receptor by using a quantitative high-throughput screening platform. Toxicology. 2017 Jun 15;385:48-58. doi: 10.1016/j.tox.2017.05.001. Epub 2017 May 4.
|
| 19 |
Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res. 2005 Apr 15;11(8):2809-21. doi: 10.1158/1078-0432.CCR-04-2187.
|
| 20 |
Aromatase inhibitors: cellular and molecular effects. J Steroid Biochem Mol Biol. 2005 May;95(1-5):83-9. doi: 10.1016/j.jsbmb.2005.04.010.
|
|
|
|
|
|
|