Details of the Drug
General Information of Drug (ID: DMTWS9E)
| Drug Name |
Apremilast
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Apremilast (USAN); CC-10004; N-[2-[1-(3-ethoxy-4-methoxy-phenyl)-2-methylsulfonyl-ethyl]-1,3-dioxo-isoindol-4-yl]acetamide | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
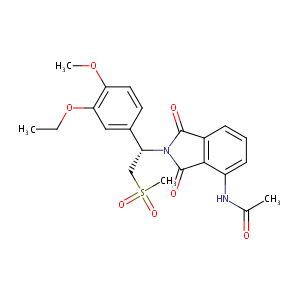 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 460.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Psoriasis vulgaris | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EA90 | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Apremilast (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7372). | ||||
|---|---|---|---|---|---|
| 2 | Apremilast FDA Label | ||||
| 3 | FDA Approved Drug Products: Otezla (apremilast) tablets for oral use | ||||
| 4 | Otezla product information | ||||
| 5 | Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor: A novel treatment option for nurse practitioners treating patients with psoriatic disease. J Am Assoc Nurse Pract. 2016 Dec;28(12):683-695. doi: 10.1002/2327-6924.12428. Epub 2016 Nov 21. | ||||
| 6 | Chavan BB, Kalariya PD, Tiwari S, Nimbalkar RD, Garg P, Srinivas R, Talluri MVNK: Identification and characterization of vilazodone metabolites in rats and microsomes by ultrahigh-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom. 2017 Dec 15;31(23):1974-1984. doi: 10.1002/rcm.7982. | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | Agreement signed with Prostagenics to develop prostate cancer treatment. Innovate Oncology, Inc. 2005. | ||||
| 9 | Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets. 2007 Mar;6(1):17-26. | ||||
| 10 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | ||||
| 11 | Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor: a novel treatment option for nurse practitioners treating patients with psoriatic disease. J Am Assoc Nurse Pract. 2016 Dec;28(12):683-695. | ||||
| 12 | Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015 Aug;40(8):495-500. | ||||
| 13 | Product Information. Otezla (apremilast). Celgene Corporation, Summit, NJ. | ||||
| 14 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 15 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 16 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 19 | Product Information. Harvoni (ledipasvir-sofosbuvir). Gilead Sciences, Foster City, CA. | ||||
| 20 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 21 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 22 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 23 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 24 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 25 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
