| 1 |
ClinicalTrials.gov (NCT04316013) Volatile Anaesthesia and Perioperative Outcomes Related to Cancer: The VAPOR-C Trial
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2623).
|
| 3 |
Lidocaine FDA Label
|
| 4 |
ClinicalTrials.gov (NCT00651313) Efficacy and Safety Study of Lidocaine Vaginal Gel for Recurrent Dysmenorrhea (Painful Periods). U.S. National Institutes of Health.
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7296).
|
| 6 |
Sevoflurane in COVID-19 ARDS (SevCov)
|
| 7 |
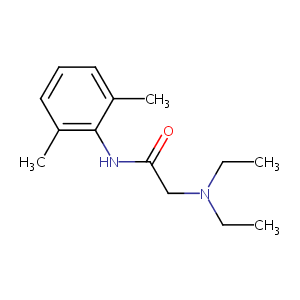
Lidocaine prevents breast cancer growth by targeting neuronatin to inhibit nerve fibers formation. J Toxicol Sci. 2021;46(7):329-339. doi: 10.2131/jts.46.329.
|
| 8 |
Mechanisms of analgesia of intravenous lidocaine. Rev Bras Anestesiol. 2008 May-Jun;58(3):280-6.
|
| 9 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 10 |
Pharmacokinetics of lidocaine hydrochloride metabolized by CYP3A4 in Chinese Han volunteers living at low altitude and in native Han and Tibetan Chinese volunteers living at high altitude. Pharmacology. 2016;97(3-4):107-13.
|
| 11 |
Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16(31):4066-218.
|
| 12 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 13 |
Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000 Aug;28(8):959-65.
|
| 14 |
Drug Interactions Flockhart Table
|
| 15 |
Insights into CYP2B6-mediated drug-drug interactions. Acta Pharm Sin B. 2016 Sep;6(5):413-425.
|
| 16 |
The effect of mild and moderate hepatic impairment on the pharmacokinetics of valdecoxib, a selective COX-2 inhibitor. Eur J Clin Pharmacol. 2005 Jun;61(4):247-56.
|
| 17 |
Evidence of significant contribution from CYP3A5 to hepatic drug metabolism. Drug Metab Dispos. 2004 Dec;32(12):1434-45. doi: 10.1124/dmd.104.001313. Epub 2004 Sep 21.
|
| 18 |
Binding of disopyramide, methadone, dipyridamole, chlorpromazine, lignocaine and progesterone to the two main genetic variants of human alpha 1-acid glycoprotein: evidence for drug-binding differences between the variants and for the presence of two separate drug-binding sites on alpha 1-acid glycoprotein. Pharmacogenetics. 1996 Oct;6(5):403-15. doi: 10.1097/00008571-199610000-00004.
|
| 19 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 20 |
Sensitivity of human dental pulp cells to eighteen chemical agents used for endodontic treatments in dentistry. Odontology. 2013 Jan;101(1):43-51.
|
| 21 |
Screening of a chemical library reveals novel PXR-activating pharmacologic compounds. Toxicol Lett. 2015 Jan 5;232(1):193-202. doi: 10.1016/j.toxlet.2014.10.009. Epub 2014 Oct 16.
|
| 22 |
Effects of capsaicin, bradykinin and prostaglandin E2 in the human skin. Br J Dermatol. 1992 Feb;126(2):111-7. doi: 10.1111/j.1365-2133.1992.tb07806.x.
|
| 23 |
[Influence of lidocaine on systemic inflammation in perioperative patients undergoing cardiopulmonary bypass]. Beijing Da Xue Xue Bao Yi Xue Ban. 2005 Dec 18;37(6):622-4.
|
| 24 |
Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010 Mar;92(3):609-18. doi: 10.2106/JBJS.H.01847.
|
| 25 |
Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19.
|
| 26 |
The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest. 2008 Feb;118(2):763-76. doi: 10.1172/JCI32751.
|
| 27 |
Studies on sulfation of synthesized metabolites from the local anesthetics ropivacaine and lidocaine using human cloned sulfotransferases. Drug Metab Dispos. 1999 Sep;27(9):1057-63.
|
| 28 |
H(1)R mediates local anesthetic-induced vascular permeability in angioedema. Toxicol Appl Pharmacol. 2020 Apr 1;392:114921. doi: 10.1016/j.taap.2020.114921. Epub 2020 Feb 12.
|
| 29 |
Lidocaine-induced Brugada syndrome phenotype linked to a novel double mutation in the cardiac sodium channel. Circ Res. 2008 Aug 15;103(4):396-404. doi: 10.1161/CIRCRESAHA.108.172619. Epub 2008 Jul 3.
|
| 30 |
Effects of sevoflurane on carrageenan- and fentanyl-induced pain hypersensitivity in Sprague-Dawley rats. Can J Anaesth. 2009 Feb;56(2):126-35.
|
| 31 |
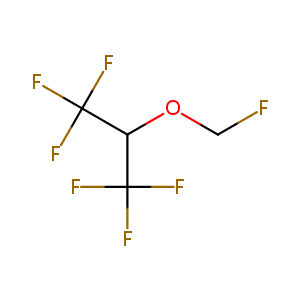
Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993 Oct;79(4):795-807.
|
| 32 |
Human kidney methoxyflurane and sevoflurane metabolism. Intrarenal fluoride production as a possible mechanism of methoxyflurane nephrotoxicity. Anesthesiology. 1995 Mar;82(3):689-99.
|
| 33 |
Inhibition of cytochrome P450 2E1 by propofol in human and porcine liver microsomes. Biochem Pharmacol. 2002 Oct 1;64(7):1151-6.
|
| 34 |
Different apoptosis ratios and gene expressions in two human cell lines after sevoflurane anaesthesia. Acta Anaesthesiol Scand. 2009 Oct;53(9):1192-9. doi: 10.1111/j.1399-6576.2009.02036.x. Epub 2009 Jun 30.
|
| 35 |
4.8% sevoflurane induces activation of autophagy in human neuroblastoma SH-SY5Y cells by the AMPK/mTOR signaling pathway. Neurotoxicology. 2022 May;90:256-264. doi: 10.1016/j.neuro.2022.04.008. Epub 2022 Apr 23.
|
| 36 |
Sevoflurane but not propofol enhances ovarian cancer cell biology through regulating cellular metabolic and signaling mechanisms. Cell Biol Toxicol. 2023 Aug;39(4):1395-1411. doi: 10.1007/s10565-022-09766-6. Epub 2022 Oct 8.
|
| 37 |
The differential cancer growth associated with anaesthetics in a cancer xenograft model of mice: mechanisms and implications of postoperative cancer recurrence. Cell Biol Toxicol. 2023 Aug;39(4):1561-1575. doi: 10.1007/s10565-022-09747-9. Epub 2022 Aug 12.
|
| 38 |
The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009 May;66(5):620-31. doi: 10.1001/archneurol.2009.48.
|
| 39 |
Sevoflurane-mediated activation of p38-mitogen-activated stresskinase is independent of apoptosis in Jurkat T-cells. Anesth Analg. 2008 Apr;106(4):1150-60, table of contents. doi: 10.1213/ane.0b013e3181683d37.
|
| 40 |
Sevoflurane-induced oxidative stress and cellular injury in human peripheral polymorphonuclear neutrophils. Food Chem Toxicol. 2006 Aug;44(8):1399-407. doi: 10.1016/j.fct.2006.03.004. Epub 2006 Mar 29.
|
| 41 |
CircRNA VIM silence synergizes with sevoflurane to inhibit immune escape and multiple oncogenic activities of esophageal cancer by simultaneously regulating miR-124/PD-L1 axis. Cell Biol Toxicol. 2022 Oct;38(5):825-845. doi: 10.1007/s10565-021-09613-0. Epub 2021 May 20.
|
|
|
|
|
|
|