| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
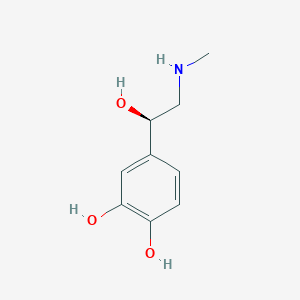
Epinephrine FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 509).
|
| 4 |
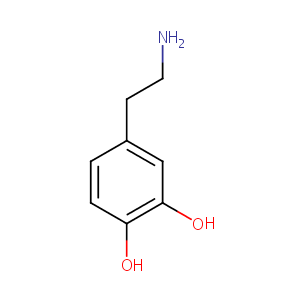
Dopamine FDA Label
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 940).
|
| 6 |
Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: involvement of beta1- and beta2-adrenergic receptors. Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G269-77.
|
| 7 |
Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006 Jun;50(8):941-52.
|
| 8 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 9 |
Steroid glucuronides: human circulatory levels and formation by LNCaP cells. J Steroid Biochem Mol Biol. 1991;40(4-6):593-8.
|
| 10 |
Crystal structure of human sulfotransferase SULT1A3 in complex with dopamine and 3'-phosphoadenosine 5'-phosphate. Biochem Biophys Res Commun. 2005 Sep 23;335(2):417-23.
|
| 11 |
Adrenal catecholamines and their metabolism in the vitamin A deficient rat. Ann Nutr Metab. 1983;27(3):220-7.
|
| 12 |
Different metabolism of norepinephrine and epinephrine by catechol-O-methyltransferase and monoamine oxidase in rats. J Pharmacol Exp Ther. 1994 Mar;268(3):1242-51.
|
| 13 |
Role of monoamine-oxidase-A-gene variation in the development of glioblastoma in males: a case control study. J Neurooncol. 2019 Nov;145(2):287-294.
|
| 14 |
Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001 Feb;59(2):393-402. doi: 10.1124/mol.59.2.393.
|
| 15 |
Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010 Dec;335(3):743-53. doi: 10.1124/jpet.110.170142. Epub 2010 Sep 21.
|
| 16 |
Epinephrine upregulates superoxide dismutase in human coronary artery endothelial cells. Free Radic Biol Med. 2001 Jan 15;30(2):148-53.
|
| 17 |
Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta. 2003 May 12;1640(2-3):137-42.
|
| 18 |
Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983 Dec 8;309(23):1414-9. doi: 10.1056/NEJM198312083092303.
|
| 19 |
A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest. 1967 Jan;46(1):86-94. doi: 10.1172/JCI105514.
|
| 20 |
Myocardial ischaemia and ventricular arrhthymias precipitated by physiological concentrations of adrenaline in patients with coronary artery disease. Br Heart J. 1992 May;67(5):419-20. doi: 10.1136/hrt.67.5.419-b.
|
| 21 |
Epinephrine facilitates the growth of T cell lymphoma by altering cell proliferation, apoptosis, and glucose metabolism. Chem Biol Interact. 2023 Jan 5;369:110278. doi: 10.1016/j.cbi.2022.110278. Epub 2022 Nov 22.
|
| 22 |
Carvedilol selectively inhibits oscillatory intracellular calcium changes evoked by human alpha1D- and alpha1B-adrenergic receptors. Cardiovasc Res. 2004 Sep 1;63(4):662-72. doi: 10.1016/j.cardiores.2004.05.014.
|
| 23 |
Carvedilol prevents epinephrine-induced apoptosis in human coronary artery endothelial cells: modulation of Fas/Fas ligand and caspase-3 pathway. Cardiovasc Res. 2000 Feb;45(3):788-94. doi: 10.1016/s0008-6363(99)00369-7.
|
| 24 |
The platelet P2Y12 receptor contributes to granule secretion through Ephrin A4 receptor. Platelets. 2012;23(8):617-25. doi: 10.3109/09537104.2011.645924. Epub 2012 Jan 24.
|
| 25 |
Hormone-sensitive lipase in human adipose tissue, isolated adipocytes, and cultured adipocytes. Pediatr Res. 1982 Dec;16(12):982-8. doi: 10.1203/00006450-198212000-00002.
|
| 26 |
Epidermal growth factor facilitates epinephrine inhibition of P2X7-receptor-mediated pore formation and apoptosis: a novel signaling network. Endocrinology. 2005 Jan;146(1):164-74. doi: 10.1210/en.2004-1026. Epub 2004 Sep 30.
|
| 27 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 28 |
Evaluation of cytogenetic and DNA damage in human lymphocytes treated with adrenaline in vitro. Toxicol In Vitro. 2015 Feb;29(1):27-33. doi: 10.1016/j.tiv.2014.08.001. Epub 2014 Aug 27.
|
| 29 |
Enzymatic characterization and interspecies difference of phenol sulfotransferases, ST1A forms. Drug Metab Dispos. 2001 Mar;29(3):274-81.
|
| 30 |
Increased arterial pressure in mice with overexpression of the ADHD candidate gene calcyon in forebrain. PLoS One. 2019 Feb 12;14(2):e0211903. doi: 10.1371/journal.pone.0211903. eCollection 2019.
|
| 31 |
Mitochondrial proteomics investigation of a cellular model of impaired dopamine homeostasis, an early step in Parkinson's disease pathogenesis. Mol Biosyst. 2014 Jun;10(6):1332-44.
|
| 32 |
Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016 Oct 1;333:193-203. doi: 10.1016/j.neuroscience.2016.07.020. Epub 2016 Jul 20.
|
| 33 |
Discovery and replication of dopamine-related gene effects on caudate volume in young and elderly populations (N=1198) using genome-wide search. Mol Psychiatry. 2011 Sep;16(9):927-37, 881. doi: 10.1038/mp.2011.32. Epub 2011 Apr 19.
|
| 34 |
The Detection of Dopamine Gene Receptors (DRD1-DRD5) Expression on Human Peripheral Blood Lymphocytes by Real Time PCR. Iran J Allergy Asthma Immunol. 2004 Dec;3(4):169-74.
|
| 35 |
Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug Metab Pharmacokinet. 2008;23(4):243-53.
|
| 36 |
SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol Aspects Med. 2013 Apr-Jun;34(2-3):360-72.
|
| 37 |
Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson disease. Proc Natl Acad Sci U S A. 2017 Mar 14;114(11):E2253-E2262.
|
| 38 |
Characterization of VNTRs Within the Entire Region of SLC6A3 and Its Association with Hypertension. DNA Cell Biol. 2017 Mar;36(3):227-236.
|
| 39 |
Modulation of CYP1A2 enzyme activity by indoleamines: inhibition by serotonin and tryptamine. Pharmacogenetics. 1998 Jun;8(3):251-8.
|
| 40 |
Pharmacogenetics of schizophrenia. Am J Med Genet. 2000 Spring;97(1):98-106.
|
| 41 |
Association between polymorphisms in catechol-O-methyltransferase (COMT) and cocaine-induced paranoia in European-American and African-American populations. Am J Med Genet B Neuropsychiatr Genet. 2011 Sep;156B(6):651-60.
|
| 42 |
Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011 Jul;1813(7):1323-32.
|
| 43 |
Molecular cloning, expression, and functional characterization of novel mouse sulfotransferases. Biochem Biophys Res Commun. 1998 Jun 29;247(3):681-6.
|
| 44 |
Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019 Jun 14;364(6445). pii: eaau6323.
|
| 45 |
Effect of penicillin-based antibiotics, amoxicillin, ampicillin, and piperacillin, on drug-metabolizing activities of human hepatic cytochromes P450. J Toxicol Sci. 2016 Feb;41(1):143-6.
|
| 46 |
Inhibition potential of 3,4-methylenedioxymethamphetamine (MDMA) and its metabolites on the in vitro monoamine oxidase (MAO)-catalyzed deamination of the neurotransmitters serotonin and dopamine. Toxicol Lett. 2016 Jan 22;243:48-55.
|
| 47 |
Functional characterization of N-octyl 4-methylamphetamine variants and related bivalent compounds at the dopamine and serotonin transporters using Ca(2+) channels as sensors. Toxicol Appl Pharmacol. 2021 May 15;419:115513. doi: 10.1016/j.taap.2021.115513. Epub 2021 Mar 27.
|
| 48 |
The effect of rare human sequence variants on the function of vesicular monoamine transporter 2. Pharmacogenetics. 2004 Sep;14(9):587-94. doi: 10.1097/00008571-200409000-00003.
|
| 49 |
Effects of dopamine on LC3-II activation as a marker of autophagy in a neuroblastoma cell model. Neurotoxicology. 2009 Jul;30(4):658-65. doi: 10.1016/j.neuro.2009.04.007. Epub 2009 May 4.
|
| 50 |
Inhibition of human glutathione S-transferases by dopamine, alpha-methyldopa and their 5-S-glutathionyl conjugates. Chem Biol Interact. 1994 Jan;90(1):87-99.
|
| 51 |
Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiatry. 2004 Apr;9(4):358-70.
|
| 52 |
Functional expression and comparative characterization of nine murine cytochromes P450 by fluorescent inhibition screening. Drug Metab Dispos. 2008 Jul;36(7):1322-31.
|
| 53 |
Induction of parkin expression in the presence of oxidative stress. Eur J Neurosci. 2006 Sep;24(5):1366-72. doi: 10.1111/j.1460-9568.2006.04998.x.
|
| 54 |
Mitochondrial UCP5 is neuroprotective by preserving mitochondrial membrane potential, ATP levels, and reducing oxidative stress in MPP+ and dopamine toxicity. Free Radic Biol Med. 2010 Sep 15;49(6):1023-35. doi: 10.1016/j.freeradbiomed.2010.06.017. Epub 2010 Jun 19.
|
| 55 |
Ligand binding and aggregation of pathogenic SOD1. Nat Commun. 2013;4:1758. doi: 10.1038/ncomms2750.
|
| 56 |
Dose-dependent separation of dopaminergic and adrenergic effects of epinine in healthy volunteers. Naunyn Schmiedebergs Arch Pharmacol. 1995 Oct;352(4):429-37. doi: 10.1007/BF00172781.
|
| 57 |
Effect of drugs interacting with the dopaminergic receptors on glucose levels and insulin release in healthy and type 2 diabetic subjects. Am J Ther. 2008 Jul-Aug;15(4):397-402. doi: 10.1097/MJT.0b013e318160c353.
|
| 58 |
Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum Mol Genet. 2004 Aug 15;13(16):1745-54. doi: 10.1093/hmg/ddh180. Epub 2004 Jun 15.
|
| 59 |
Resveratrol protects SH-SY5Y neuroblastoma cells from apoptosis induced by dopamine. Exp Mol Med. 2007 Jun 30;39(3):376-84. doi: 10.1038/emm.2007.42.
|
| 60 |
Characterizing fucoxanthin as a selective dopamine D(3)/D(4) receptor agonist: Relevance to Parkinson's disease. Chem Biol Interact. 2019 Sep 1;310:108757. doi: 10.1016/j.cbi.2019.108757. Epub 2019 Jul 16.
|
| 61 |
Dopamine down-regulation of protein L-isoaspartyl methyltransferase is dependent on reactive oxygen species in SH-SY5Y cells. Neuroscience. 2014 May 16;267:263-76. doi: 10.1016/j.neuroscience.2014.03.001. Epub 2014 Mar 12.
|
| 62 |
Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases. Biochem Biophys Res Commun. 2000 Jan 7;267(1):80-4. doi: 10.1006/bbrc.1999.1935.
|
| 63 |
Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004 Mar 3;24(9):2212-25. doi: 10.1523/JNEUROSCI.4847-03.2004.
|
| 64 |
Expression of tyrosine hydroxylase increases the resistance of human neuroblastoma cells to oxidative insults. Toxicol Sci. 2010 Jan;113(1):150-7. doi: 10.1093/toxsci/kfp245. Epub 2009 Oct 8.
|
| 65 |
G209A mutant alpha synuclein expression specifically enhances dopamine induced oxidative damage. Neurochem Int. 2004 Oct;45(5):669-76. doi: 10.1016/j.neuint.2004.03.029.
|
| 66 |
Administration of secretin for autism alters dopamine metabolism in the central nervous system. Brain Dev. 2006 Mar;28(2):99-103. doi: 10.1016/j.braindev.2005.05.005. Epub 2005 Sep 15.
|
|
|
|
|
|
|