Details of the Drug
General Information of Drug (ID: DM3KJBC)
| Drug Name |
Epinephrine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ADROP; Adnephrine; Adrenal; Adrenalin; Adrenalina; Adrenaline; Adrenalinum; Adrenamine; Adrenan; Adrenapax; Adrenasol; Adrenatrate; Adrenine; Adrenodis; Adrenohorma; Adrenosan; Adrenutol; Adrin; Adrine; Antiasthmatique; Asthmanefrin; Astmahalin; Astminhal; Balmadren; Bernarenin; Biorenine; Bosmin; Brevirenin; Bronkaid; Chelafrin; Corisol; Drenamist; Dylephrin; Epifrin; Epiglaufrin; Epinefrin; Epinefrina; Epinephran; Epinephrin; Epinephrinum; Epipen; Epirenamine; Epirenan; Epirenin; Epitrate; Eppy; Esphygmogenina; Exadrin; Glaucon; Glaucosan; Glauposine; Glycirenan; Haemostasin; Haemostatin; Hektalin; Hemisine; Hemostasin; Hemostatin; Hypernephrin; Hyporenin; IOP; Intranefrin; Iontocaine; Isoptoepinal; Kidoline; Levoadrenaline; Levoepinephrine; Levorenen; Levorenin; Levorenine; Lyophrin; Metanephrin; Methylaminoethanolcatechol; Methylarterenol; Micronefrin; Micronephrine; Mucidrina; Myosthenine; Mytrate; Nephridine; Nieraline; Paranephrin; Primatene; Racepinephrine; Renagladin; Renaglandin; Renaglandulin; Renaleptine; Renalina; Renoform; Renostypricin; Renostypticin; Renostyptin; Scurenaline; Septocaine; Simplene; Sindrenina; Soladren; Sphygmogenin; Stryptirenal; Styptirenal; Supracapsulin; Supradin; Supranefran; Supranephrane; Supranephrine; Supranol; Suprarenaline; Suprarenin; Suprel; Surenine; Surrenine; Susphrine; Takamina; Takamine; Tokamina; Tonogen; Twinject; Vaponefrin; Vasoconstrictine; Vasoconstrictor; Vasodrine; Vasoton; Vasotonin; ADR ADRENALINE; Adrenalin in Oil; Adrenalina [DCIT]; Asmatane Mist; Asthma meter mist; Asthmahaler Mist; Bronkaid Mist; Bronkaid Suspension Mist; Bupivacaine Hcl and Epinephrine; Citanest Forte; EPI E Z PEN JR; EPIPEN E Z PEN; EPIPEN JR; Epi EZ Pen Jr; Epinefrin [Czech]; Epinephrine hydrochloride; Epipen EZ Pen; Primatene Mist; Racemic Epinephrine; Sympathin I; Adrenalin (TN); Adrenalin-Medihaler; Adrenaline (JP15); Adrenaline/Epinephrine; Ana-Guard; Asthma-nefrin; D-Adrenaline; D-Epifrin; D-Epinephrine; Dyspne-Inhal; Epinefrina [INN-Spanish]; Epinephrine (USP); Epinephrinum [INN-Latin]; Epipen (TN); Epipen Auto-Injector; Epipen Jr.; L-Adrenalin; L-Adrenaline; L-Adrenaline Base; L-Epinehphrine; L-Epinephine; L-Epirenamine; L-Methylaminoethanolcatechol; L-epinephrine; Levo-Methylaminoethanolcatechol; Medihaler-Epi; R-Adrenaline; Sus-Phrine; Twinject 0.15; Twinject 0.3; Twinject 0.30; Epinephrine (USP/INN); Epinephrine [USAN:INN:JAN]; Epipen Jr. Auto-Injector; L-Epinephrine (synthetic); SUS-PHRINE SULFITE-FREE; R-(-)-Epinephrine; L-1-(3,4-Dihydroxyphenyl)-2-methylaminoethanol; (-)-(R)-Epinephrine; (-)-3,4-Dihydroxy-alpha-((methylamino)methyl)benzyl alcohol; (-)-Adrenalin; (-)-Adrenaline; (-)-R-Epinephrine; (R)-(-)-Adnephrine; (R)-(-)-Adrenaline; (R)-(-)-Epinephrine; (R)-(-)-Epirenamine; (R)-4-[1-Hydroxy-2-(methylamino)ethyl]-1,2-benzenediol; (R)-Adrenaline; (R)-Epinephrine; 1-1-(3,4-Dihydroxyphenyl)-2-methylaminoethanol; 1-Adrenalin; 1-Epinephrine; 4-(1-Hydroxy-2-(methylamino)ethyl)-1,2-benzenediol; 4-(1-hydroxy-2-methylamino-ethyl)benzene-1,2-diol; 4-[(1R)-1-Hydroxy-2-(methylamino)ethyl]-1,2-benzenediol; 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Vasoconstrictor Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
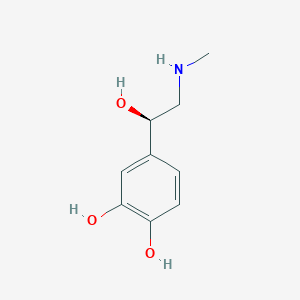 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 183.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Epinephrine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Epinephrine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 509). | ||||
| 3 | Medicines UK document | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 6 | Adrenergic activation of electrogenic K+ secretion in guinea pig distal colonic epithelium: involvement of beta1- and beta2-adrenergic receptors. Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G269-77. | ||||
| 7 | Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006 Jun;50(8):941-52. | ||||
| 8 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | ||||
| 9 | Steroid glucuronides: human circulatory levels and formation by LNCaP cells. J Steroid Biochem Mol Biol. 1991;40(4-6):593-8. | ||||
| 10 | Crystal structure of human sulfotransferase SULT1A3 in complex with dopamine and 3'-phosphoadenosine 5'-phosphate. Biochem Biophys Res Commun. 2005 Sep 23;335(2):417-23. | ||||
| 11 | Role of monoamine-oxidase-A-gene variation in the development of glioblastoma in males: a case control study. J Neurooncol. 2019 Nov;145(2):287-294. | ||||
| 12 | Different metabolism of norepinephrine and epinephrine by catechol-O-methyltransferase and monoamine oxidase in rats. J Pharmacol Exp Ther. 1994 Mar;268(3):1242-51. | ||||
| 13 | Adrenal catecholamines and their metabolism in the vitamin A deficient rat. Ann Nutr Metab. 1983;27(3):220-7. | ||||
| 14 | Carvedilol selectively inhibits oscillatory intracellular calcium changes evoked by human alpha1D- and alpha1B-adrenergic receptors. Cardiovasc Res. 2004 Sep 1;63(4):662-72. doi: 10.1016/j.cardiores.2004.05.014. | ||||
| 15 | Epinephrine facilitates the growth of T cell lymphoma by altering cell proliferation, apoptosis, and glucose metabolism. Chem Biol Interact. 2023 Jan 5;369:110278. doi: 10.1016/j.cbi.2022.110278. Epub 2022 Nov 22. | ||||
| 16 | Myocardial ischaemia and ventricular arrhthymias precipitated by physiological concentrations of adrenaline in patients with coronary artery disease. Br Heart J. 1992 May;67(5):419-20. doi: 10.1136/hrt.67.5.419-b. | ||||
| 17 | Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta. 2003 May 12;1640(2-3):137-42. | ||||
| 18 | Carvedilol prevents epinephrine-induced apoptosis in human coronary artery endothelial cells: modulation of Fas/Fas ligand and caspase-3 pathway. Cardiovasc Res. 2000 Feb;45(3):788-94. doi: 10.1016/s0008-6363(99)00369-7. | ||||
| 19 | Evaluation of cytogenetic and DNA damage in human lymphocytes treated with adrenaline in vitro. Toxicol In Vitro. 2015 Feb;29(1):27-33. doi: 10.1016/j.tiv.2014.08.001. Epub 2014 Aug 27. | ||||
| 20 | Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001 Feb;59(2):393-402. doi: 10.1124/mol.59.2.393. | ||||
| 21 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 22 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 23 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 24 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 25 | Barthel W, Glusa E, Koth W "Interactions of dihydroergotamine with etilefrine in human leg veins in vitro and in situ." Int J Clin Pharmacol Ther Toxicol 25 (1987): 63-9. [PMID: 2881898] | ||||
| 26 | Product Information. Meridia (sibutramine). Knoll Pharmaceutical Company, Whippany, NJ. | ||||
| 27 | Cusson JR, Goldenberg E, Larochelle P "Effect of a novel monoamine-oxidase inhibitor, moclobemide on the sensitivity to intravenous tyramine and norepinephrine in humans." J Clin Pharmacol 31 (1991): 462-7. [PMID: 2050833] | ||||
| 28 | Illi A, Sundberg S, Ojala-Karlsson P, Korhonen P, Scheinin M, Gordin A "The effect of entacapone on the disposition and hemodynamic effects of intravenous isoproterenol and epinephrine." Clin Pharmacol Ther 58 (1995): 221-7. [PMID: 7648772] | ||||
