Details of the Drug
General Information of Drug (ID: DMYBV3G)
| Drug Name |
Paricalcitol
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Paracalcin; Zemplar; Abbott brand of paricalcitol; Paricalcitol [USAN]; Ab 122358; ABT-358; Zemplar (TN); Paricalcitol (USAN/INN); Paricalcitol, 19-nor-(OH)2-vitD2, paracalcin; (1R,3R)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]cyclohexane-1,3-diol; (1R,3R,7E)-17beta-[(2R,3E,5S)-6-hydroxy-5,6-dimethylhept-3-en-2-yl]-9,10-secoestra-5,7-diene-1,3-diol; (1alpha.3beta,7E,22E)-19-Nor-9,10-secoergosta-5,7,22-triene-1,3,25-triol; (7E,22E)-19-Nor-9,10-secoergosta-5,7,22-triene-1alpha,3beta,25-triol; 19-Nor-1,25-(OH)2D2; 19-Nor-1-alpha,25-dihydroxyvitamin D2; 19-Nor-1alpha,25-dihydroxyvitamin D2; 19-Nor-9,10-secoergosta-5,7,22-triene-1,3,25-triol,(1alpha,3beta,7E,22E)
|
|||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Parasympathomimetics
|
|||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||
| Structure |
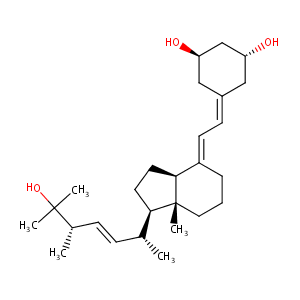 |
|||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 416.6 | ||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5 | |||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | |||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hyperparathyroidism | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A51 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Paricalcitol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2791). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 7 | New acquisitions in therapy of secondary hyperparathyroidism in chronic kidney disease and peritoneal dialysis patients: role of vitamin D receptor... Contrib Nephrol. 2009;163:219-226. | ||||
| 8 | Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol Pharmacol. 2001 Dec;60(6):1399-406. | ||||
| 9 | 19-nor-1alpha,25-dihydroxyvitamin D(2) (paricalcitol): effects on clonal proliferation, differentiation, and apoptosis in human leukemic cell lines. J Cancer Res Clin Oncol. 2003 Jan;129(1):35-42. doi: 10.1007/s00432-002-0405-7. Epub 2003 Feb 12. | ||||
| 10 | Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003 Apr;63(4):1483-90. doi: 10.1046/j.1523-1755.2003.00878.x. | ||||
| 11 | Spotlight on paricalcitol in secondary hyperparathyroidism. Treat Endocrinol. 2005;4(3):185-6. doi: 10.2165/00024677-200504030-00007. | ||||
| 12 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 13 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 14 | Product Information. Hectorol (doxercalciferol). Genzyme Corporation, Cambridge, MA. | ||||
| 15 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 16 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 17 | Product Information. Retevmo (selpercatinib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 18 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 19 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 20 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 21 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 22 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 23 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 24 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 25 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
