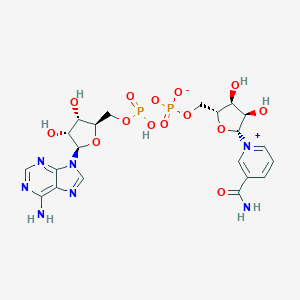
| Synonyms |
NICOTINAMIDE-ADENINE-DINUCLEOTIDE (ACIDIC FORM); AC1NRCGS; [[(2R,3S,4R,5R)-5-(6-amino-7H-purin-9-ium-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate
|
| Chemical Identifiers |
- Formula
- C21H27N7O14P2
- IUPAC Name
[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoylpyridin-1-ium-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl phosphate - Canonical SMILES
-
C1=CC(=C[N+](=C1)[C@H]2[C@@H]([C@@H]([C@H](O2)COP(=O)([O-])OP(=O)(O)OC[C@@H]3[C@H]([C@H]([C@@H](O3)N4C=NC5=C(N=CN=C54)N)O)O)O)O)C(=O)N
- InChI
-
InChI=1S/C21H27N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1-4,7-8,10-11,13-16,20-21,29-32H,5-6H2,(H5-,22,23,24,25,33,34,35,36,37)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1
- InChIKey
-
BAWFJGJZGIEFAR-NNYOXOHSSA-N
|