Details of the Drug
General Information of Drug (ID: DMC1TEV)
| Drug Name |
Testosterone cypionate
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Testodrin prolongatum; Testosterone 17beta-cyclopentylpropionate; Testosterone 17beta-cypionate; Testosterone cyclopentanepropionate; Testosterone cyclopentylpropionate; Testosterone cypionate [USP]; Testandrone; Testiculosterone; Testim; Testobase; Testoderm; Testogel; Testopropon; Testosteroid; Testosteron; Testosterona; Testosteronum; Testostosterone; Testoviron; Testoviron Schering; Testoviron T; Testrone; Testryl; Virormone; Virosterone; testosterone; trans-Testosterone; 58-22-0; AndroGel; Androderm; Androlin; Andronaq; Andropatch; Andrusol; Cristerona T; Cristerone T; Homosteron; Homosterone; LibiGel; Malerone; Mertestate; Neotestis; Oreton; Orquisteron; Perandren; Primotest; Primoteston; Relibra; Sustanon; Sustanone; Synandrol F; Teslen; UNII-M0XW1UBI14; depAndro 100; depAndro 200; 58-20-8; Andro-Cyp; Androst-4-en-3-one, 17-(3-cyclopentyl-1-oxopropoxy)-, (17b)-; CHEBI:9463; DEPO; Dep-Test; Depo-Testadiol; Depo-Testosterone; Depotest; Depovirin; Durandro; Jectatest; M0XW1UBI14; Malogen CYP; NSC 9157; Pertestis; T-Ionate-P.A; TESTOSTERONE CYPIONATE
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| Structure |
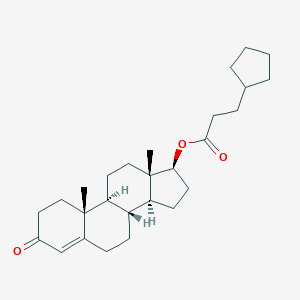 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 412.6 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 6.4 | |||||
| Rotatable Bond Count (rotbonds) | 5 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
