Details of the Drug
General Information of Drug (ID: DM1DP7T)
| Drug Name |
Levonorgestrel
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
norgestrel; 797-63-7; D-Norgestrel; (-)-Norgestrel; Microval; Levonova; Postinor; Mirena; Ovrette; Neogest; Jadelle; Plan B; NORPLANT; Norplant 2; Microlution; Follistrel; Trivora; Monovar; Triciclor; Microgyn; Ovranette; Triagynon; Microlut; Nordet; Trigoa; 18-Methylnorethisterone; Levonorgestrelum; Microgynon CD; Microgest ED; Norplant II; Ovral-Lo; Logynon ED; Levlen ED; Trinordiol 28; Trinordiol 21; Microgynon 28; Microgynon 21; d(-)-Norgestrel; Neogynon 21; Monofeme 28; Nordette 28; Minivlar 30; Stediril 30; Nordette 21; Trifeme; Capronor; DNorgestrel; Levlen; Levonelle; Methylnorethindrone; Microluton; NorLevo; Norgeston; Norgestrel; Norgestrelum; Preven; Tetragynon; LD norgestrel [French]; Ld norgestrel; Levonorgestrel implants; Norgestrel [Progestins]; Norplant System in Plastic Container; Triquilar ED; Microgynon 30 ED; SH 70850; SH 850; Trifeme 28; Triphasil 21; Triphasil 28; Wy 3707; Alpha-Norgestrel; Component of Lo/ovral; Dl-Norgestrel; FH 122-A; LO/Ovral; Levonorgestrelum [INN-Latin]; Levora-21; Levora-28; Mirena (TN); Norgestrelum [INN-Latin]; Norplant (TN); Norplant-2; Ovoplex 30-150; Ovrette (TN); Postinor-2; Rigevidon 21+7; SOH-075; Tri-Levlen 21; Wy-3707; Wy-5104; E-Gen-C; Levonorgestrel [USAN:INN:BAN]; D(-)-Norgestrel; Levonorgestrel (JAN/USP/INN); Norgestrel (JP15/USP/INN); Norgestrel [USAN:BAN:INN:JAN]; Norgestrel [USAN:INN:BAN:JAN]; D-(-)-Norgestrel; Levonelle, D-Norgestrel, Levonova, Levonorgestrel; Norgestrel-(-)-D; Dl-13-beta-Ethyl-17-alpha-ethynyl-19-nortestosterone; 13-BETA-ETHYL-17-ALPHA-ETHYNYL-17-BETA-HYDROXYGON-4-EN-3-ONE; 13-Ehyl-17alpha-ethynyl-17-hydroxygon-4-en-3-one; 13-Ethyl-17-alpha-ethynyl-17-beta-hydroxy-4-gonen-3-one; 13-Ethyl-17-alpha-ethynylgon-4-en-17-beta-ol-3-one; 13-Ethyl-17alpha-ethynylgon-4-en-17beta-ol-3-one; 13-beta-Ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one; 13beta-Ethyl-17alpha-ethynyl-17beta-hydroxygon-4-en-3-one; 17-Ethynyl-18-methyl-19-nortestosterone; 17-alpha-Ethynyl-13-ethyl-19-nortestosterone; 17alpha-Ethynyl-13-ethyl-19-nortestosterone; 17alpha-Ethynyl-13beta-ethyl-3-oxo-4-estren-17beta-ol; 17alpha-Ethynyl-17-hydroxy-18-methylestr-4-en-3-one; 17alpha-Ethynyl-18-homo-19-nor-testosterone; 17alpha-Ethynyl-18-homo-19-nortestosterone; 17alpha-ethynyl-17beta-hydroxy-18a-homoestr-4-en-3-one; 18,19-Dinor-4-pregnen-20-yn-3-one; 18-Methyl-17-alpha-ethynyl-19-nortestosterone; 72-HOURS
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Contraceptive Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
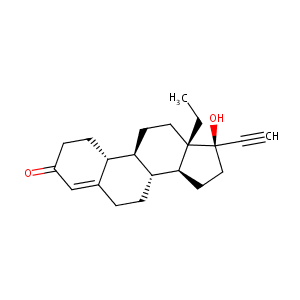 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 312.4 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.3 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Atypical endometrial hyperplasia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Levonorgestrel (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Levonorgestrel FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2881). | ||||
| 3 | Norplant FDA label | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 6 | Natavio M, Stanczyk FZ, Molins EAG, Nelson A, Jusko WJ: Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019 May;99(5):306-311. doi: 10.1016/j.contraception.2019.01.003. Epub 2019 Jan 28. | ||||
| 7 | Levonorgestrel FDA label | ||||
| 8 | Mechanism of action of levonorgestrel emergency contraception. Linacre Q. 2015 Feb;82(1):18-33. doi: 10.1179/2050854914Y.0000000026. | ||||
| 9 | Hammer HF, Hammer J, Gasche C: [Polyethylene glycol (Macrogol)--an overview of its use in diagnosis and therapy of gastrointestinal diseases]. Wien Klin Wochenschr. 2000 Jan 28;112(2):53-60. | ||||
| 10 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 11 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 12 | Met909 plays a key role in the activation of the progesterone receptor and also in the high potency of 13-ethyl progestins. Mol Pharmacol. 2009 Jun;75(6):1317-24. | ||||
| 13 | Progestins as inhibitors of the human 20-ketosteroid reductases, AKR1C1 and AKR1C3. Chem Biol Interact. 2011 May 30;191(1-3):227-33. | ||||
| 14 | Effect of menstrual cycle and hormonal treatment on ki-67 and bcl-2 expression and adenomyosis. Gynecol Endocrinol. 2005 Mar;20(3):127-31. doi: 10.1080/09513590400021086. | ||||
| 15 | P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res. 2004 Jul;21(7):1284-93. | ||||
| 16 | Study of low-density lipoprotein receptor regulation by oral (steroid) contraceptives: desogestrel, levonorgestrel and ethinyl estradiol in JEG-3 cell line and placental tissue. Contraception. 2007 Oct;76(4):297-305. doi: 10.1016/j.contraception.2007.06.011. Epub 2007 Sep 14. | ||||
| 17 | Relative progestational and androgenic activity of four progestins used for male hormonal contraception assessed in vitro in relation to their ability to suppress LH secretion in the castrate male rat. Mol Cell Endocrinol. 2010 Oct 26;328(1-2):16-21. doi: 10.1016/j.mce.2010.06.010. Epub 2010 Jun 25. | ||||
| 18 | Effects of natural products and nutraceuticals on steroid hormone-regulated gene expression. Clin Chim Acta. 2001 Oct;312(1-2):213-9. doi: 10.1016/s0009-8981(01)00626-x. | ||||
| 19 | Serum distribution of the major metabolites of norgestimate in relation to its pharmacological properties. Contraception. 2003 Feb;67(2):93-9. doi: 10.1016/s0010-7824(02)00473-0. | ||||
| 20 | [Effect of nylestriol and levonorgestrel on the expression of Opg/OPGL in human osteosarcoma MG-63 cell lines]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004 Dec;29(6):667-70. | ||||
| 21 | Product Information. Myalept (metreleptin). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 22 | Glazer NB, Cheatham WW "Thiazolidinediones for type 2 diabetes - No evidence exists that pioglitazone induces hepatic cytochrome P450 isoform CYP3A4." Br Med J 322 (2001): 235-6. [PMID: 11159615] | ||||
| 23 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 24 | Baciewicz AM "Oral contraceptive drug interactions." Ther Drug Monit 7 (1985): 26-35. [PMID: 2859674] | ||||
| 25 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 26 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 27 | Back DJ, Breckenridge AM, Crawford FE, MacIver M, Orne ML, Rowe PH "Interindividual variation and drug interactions with hormonal steroid contraceptives." Drugs 21 (1981): 46-61. [PMID: 7009137] | ||||
| 28 | Devenport MH, Crook D, Wynn V, Lees LJ "Metabolic effects of low-dose fluconazole in healthy female users and non-users of oral contraceptives." Br J Clin Pharmacol 27 (1989): 851-9. [PMID: 2547410] | ||||
| 29 | Gardner MJ, Tornatore KM, Jusko WJ, Kanarkowski R "Effects of tobacco smoking and oral contraceptive use on theophylline disposition." Br J Clin Pharmacol 16 (1983): 271-80. [PMID: 6626419] | ||||
| 30 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 31 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 32 | Product Information. Idhifa (enasidenib). Celgene Corporation, Summit, NJ. | ||||
| 33 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 34 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 35 | Product Information. Lipitor (atorvastatin). Parke-Davis, Morris Plains, NJ. | ||||
| 36 | Faculty of Sexual & Reproductive Healthcare "FSRH Clinical Guidance: Drug Interactions with Hormonal Contraception.". | ||||
| 37 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 38 | Hobbes J, Boutagy J, Shenfield GM "Interactions between ethanol and oral contraceptive steroids." Clin Pharmacol Ther 38 (1985): 371-80. [PMID: 4042520] | ||||
| 39 | Product Information. Serzone (nefazodone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 40 | Laine K, Anttila M, Helminen A, Karnani H, Huupponen R "Dose linearity study of selegiline pharmacokinetics after oral administration: evidence for strong drug interaction with female sex steroids." Br J Clin Pharmacol 47 (1999): 249-54. [PMID: 10215747] | ||||
| 41 | Back DJ, Grimmer SF, Orme ML, Proudlove D, Mann RD, Breckenridge AM "Evaluation of Committee on Safety of Medicines yellow card reports on oral contraceptive-drug interactions with anticonvulsants and antibiotics." Br J Clin Pharmacol 25 (1988): 527-32. [PMID: 3408633] | ||||
| 42 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 43 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 44 | Back DJ, Breckenridge AM, Crawford F, et al. "The effect of rifampicin on norethisterone pharmacokinetics." Eur J Clin Pharmacol 15 (1979): 193-7. [PMID: 37091] | ||||
| 45 | Archer JS, Archer DF "Oral contraceptive efficacy and antibiotic interaction: A myth debunked." J Am Acad Dermatol 46 (2002): 917-23. [PMID: 12063491] | ||||
| 46 | Barry M, Mulcahy F, Merry C, Gibbons S, Back D "Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection." Clin Pharmacokinet 36 (1999): 289-304. [PMID: 10320951] | ||||
| 47 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 48 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 49 | Product Information. Reyataz (atazanavir). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 50 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 51 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 52 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 53 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 54 | Product Information. Impavido (miltefosine). Paladin Therapeutics Inc, Wilmington, DE. | ||||
| 55 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 56 | Faculty of Sexual & Reproductive Healthcare "FSRH Clinical Guidance: Drug Interactions with Hormonal Contraception.". | ||||
| 57 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 58 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 59 | Product Information. Desferal (deferoxamine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 60 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 61 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 62 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 63 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 64 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 65 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 66 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 67 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 68 | Granfors MT, Backman JT, Laitila J, Neuvonen PJ "Oral contraceptives containing ethinyl estradiol and gestodene markedly increase plasma concentrations and effects of tizanidine by inhibiting cytochrome P450 1A2." Clin Pharmacol Ther 78 (2005): 400-11. [PMID: 16198659] | ||||
