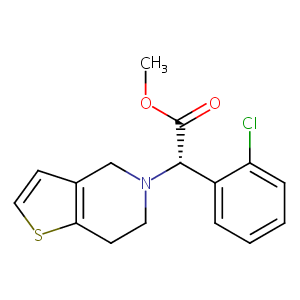
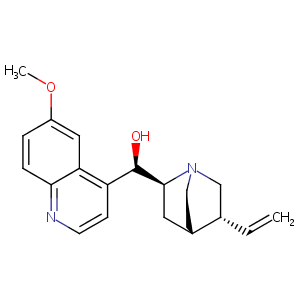
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Clopidogrel FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7150).
|
| 4 |
Preventing Cardiac Complication of COVID-19 Disease With Early Acute Coronary Syndrome Therapy: A Randomised Controlled Trial. (C-19-ACS)
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2510).
|
| 6 |
P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med. 2003 May;3(2):113-22.
|
| 7 |
Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006 Nov;80(5):486-501.
|
| 8 |
Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675.
|
| 9 |
Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet. 2015 Feb;54(2):147-66.
|
| 10 |
Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007 May;81(5):735-41.
|
| 11 |
Clopidogrel pathway. Pharmacogenet Genomics. 2010 Jul;20(7):463-5.
|
| 12 |
Impact of the CYP2C19 gene polymorphism on clopidogrel personalized drug regimen and the clinical outcomes. Clin Lab. 2016 Sep 1;62(9):1773-1780.
|
| 13 |
Identification of novel agonists by high-throughput screening and molecular modelling of human constitutive androstane receptor isoform 3. Arch Toxicol. 2019 Aug;93(8):2247-2264. doi: 10.1007/s00204-019-02495-6. Epub 2019 Jul 16.
|
| 14 |
Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010 Jan;38(1):92-9. doi: 10.1124/dmd.109.029132.
|
| 15 |
Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ. 2006 Jun 6;174(12):1715-22. doi: 10.1503/cmaj.060664.
|
| 16 |
Platelet reactivity and clopidogrel resistance are associated with the H2 haplotype of the P2Y12-ADP receptor gene. Int J Cardiol. 2009 Apr 17;133(3):341-5. doi: 10.1016/j.ijcard.2007.12.118. Epub 2008 May 15.
|
| 17 |
Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013 Sep;94(3):317-23. doi: 10.1038/clpt.2013.105. Epub 2013 May 22.
|
| 18 |
Angiogenesis inhibitor SR 25989 upregulates thrombospondin-1 expression in human vascular endothelial cells and foreskin fibroblasts. Biol Cell. 1997 Jul;89(4):295-307.
|
| 19 |
Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167.
|
| 20 |
Clopidogrel increases expression of chemokines in peripheral blood mononuclear cells in patients with coronary artery disease: results of a double-blind placebo-controlled study. J Thromb Haemost. 2006 Oct;4(10):2140-7. doi: 10.1111/j.1538-7836.2006.02131.x. Epub 2006 Jul 17.
|
| 21 |
Differential effect of clopidogrel and aspirin on the release of BDNF from platelets. J Neuroimmunol. 2011 Sep 15;238(1-2):104-6. doi: 10.1016/j.jneuroim.2011.06.015. Epub 2011 Jul 31.
|
| 22 |
Attenuated expression of the tight junction proteins is involved in clopidogrel-induced gastric injury through p38 MAPK activation. Toxicology. 2013 Feb 8;304:41-8. doi: 10.1016/j.tox.2012.11.020. Epub 2012 Dec 7.
|
| 23 |
PAR-1 genotype influences platelet aggregation and procoagulant responses in patients with coronary artery disease prior to and during clopidogrel therapy. Platelets. 2005 Sep;16(6):340-5. doi: 10.1080/00207230500120294.
|
| 24 |
Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011 Jan;17(1):110-6. doi: 10.1038/nm.2281. Epub 2010 Dec 19.
|
| 25 |
Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007 Dec;5(12):2429-36. doi: 10.1111/j.1538-7836.2007.02775.x. Epub 2007 Sep 26.
|
| 26 |
Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol. 2007 Sep;72(3):686-94. doi: 10.1124/mol.107.036889. Epub 2007 May 30.
|
| 27 |
High loading dose of clopidogrel is unable to satisfactorily inhibit platelet reactivity in patients with glycoprotein IIIA gene polymorphism: a genetic substudy of PRAGUE-8 trial. Blood Coagul Fibrinolysis. 2009 Jun;20(4):257-62. doi: 10.1097/mbc.0b013e328325455b.
|
| 28 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 29 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 30 |
Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997 Jul;16(7):871-81.
|
| 31 |
Comparison of type I and type II organic cation transport by organic cation transporters and organic anion-transporting polypeptides. J Pharmacol Exp Ther. 2001 Jul;298(1):110-5.
|
| 32 |
Na(+)-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther. 1999 Nov;291(2):778-84.
|
| 33 |
Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther. 1999 May;289(2):768-73.
|
| 34 |
The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res. 2016 Jan 4;44(D1):D372-9. (ID: 2.A.22.3.1)
|
| 35 |
Quinine induced simvastatin toxicity through cytochrome inhibition - a case report. BMC Geriatr. 2016 Oct 1;16(1):168.
|
| 36 |
The roles of cytochrome P450 3A4 and 1A2 in the 3-hydroxylation of quinine in vivo. Clin Pharmacol Ther. 1999 Nov;66(5):454-60.
|
| 37 |
Inhibition of cytochrome P450 2D6: structure-activity studies using a series of quinidine and quinine analogues. Chem Res Toxicol. 2003 Apr;16(4):450-9.
|
| 38 |
Drug Interactions Flockhart Table
|
| 39 |
Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016 Jan;68(1):168-241.
|
| 40 |
Identification of human cytochrome P450 isoforms involved in the 3-hydroxylation of quinine by human live microsomes and nine recombinant human cytochromes P450. J Pharmacol Exp Ther. 1996 Dec;279(3):1327-34.
|
| 41 |
Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001 Feb;120(2):525-33.
|
| 42 |
Application of higher throughput screening (HTS) inhibition assays to evaluate the interaction of antiparasitic drugs with cytochrome P450s. Drug Metab Dispos. 2001 Jan;29(1):30-5.
|
| 43 |
Cytochrome P450 1A1/2 induction by antiparasitic drugs: dose-dependent increase in ethoxyresorufin O-deethylase activity and mRNA caused by quinine, primaquine and albendazole in HepG2 cells. Eur J Clin Pharmacol. 2002 Nov;58(8):537-42.
|
| 44 |
Involvement of aryl hydrocarbon receptor in the cytotoxicity of corannulene and its derivatives. Toxicol Lett. 2020 Mar 15;321:114-121. doi: 10.1016/j.toxlet.2019.12.012. Epub 2019 Dec 9.
|
| 45 |
Stereoselective transport of hydrophilic quaternary drugs by human MDR1 and rat Mdr1b P-glycoproteins. Br J Pharmacol. 2002 Apr;135(7):1685-94. doi: 10.1038/sj.bjp.0704620.
|
| 46 |
Inhibition of glutathione S-transferases by antimalarial drugs possible implications for circumventing anticancer drug resistance. Int J Cancer. 2002 Feb 10;97(5):700-5.
|
| 47 |
Voltage-dependent profile of human ether-a-go-go-related gene channel block is influenced by a single residue in the S6 transmembrane domain. Mol Pharmacol. 2003 May;63(5):1051-8. doi: 10.1124/mol.63.5.1051.
|
| 48 |
Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J Biol Chem. 2001 May 18;276(20):17387-94. doi: 10.1074/jbc.M101319200. Epub 2001 Feb 26.
|
| 49 |
Expression of Organic Anion Transporting Polypeptide 1A2 in Red Blood Cells and Its Potential Impact on Antimalarial Therapy. Drug Metab Dispos. 2016 Oct;44(10):1562-8. doi: 10.1124/dmd.116.069807. Epub 2016 Aug 8.
|
|
|
|
|
|
|