Details of the Drug
General Information of Drug (ID: DM1BV7W)
| Drug Name |
Aliskiren
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Rasilez; SPP 100; Rasilez (TN); Tekturna (TN); Aliskiren (USAN/INN); (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methyl-2-(propan-2-yl)nonanamide; (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-2-propan-2-ylnonanamide; (2S,4S,5S,7S)-N-(2-Carbamoyl-2-methylpropyl)-5-amino-4-hydroxy-2,7-diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]octanamide; Octanamide, .delta.-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-.gamma.-hydroxy-4-methoxy-3-(3-methoxypropoxy)-.alpha.,.zeta.-bis(1-methylethyl)-, [.alpha.S-(alpha.R*,.gamma.R*,.delta.R*,.zeta.R*)]
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
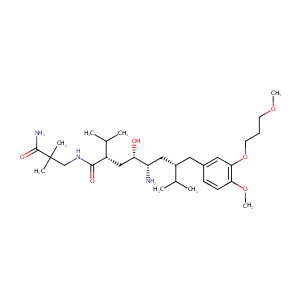 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 551.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 19 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Aliskiren (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4812). | ||||
|---|---|---|---|---|---|
| 2 | FDA Drug Approval Package: Zovirax Oral Capsules, Oral Tablets, Oral Suspension | ||||
| 3 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 4 | FDA Approved Products: Tekturna (Aliskiren) oral tablets and pellets | ||||
| 5 | Vaidyanathan S, Jarugula V, Dieterich HA, Howard D, Dole WP: Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clin Pharmacokinet. 2008;47(8):515-31. | ||||
| 6 | Absorption, distribution, metabolism, and elimination of the direct renin inhibitor aliskiren in healthy volunteers. Drug Metab Dispos. 2007 Aug;35(8):1418-28. doi: 10.1124/dmd.106.013797. Epub 2007 May 17. | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Comparative effects of aliskiren-based and ramipril-based therapy on the renin system during long-term (6 months) treatment and withdrawal in patie... J Renin Angiotensin Aldosterone Syst. 2009 Sep;10(3):157-67. | ||||
| 9 | Orange and apple juice greatly reduce the plasma concentrations of the OATP2B1 substrate aliskiren. Br J Clin Pharmacol. 2011 May;71(5):718-26. | ||||
| 10 | Effects of the inhibition of intestinal P-glycoprotein on aliskiren pharmacokinetics in cynomolgus monkeys. Biopharm Drug Dispos. 2015 Jan;36(1):15-33. | ||||
| 11 | Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clin Pharmacokinet. 2008;47(8):515-31. | ||||
| 12 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 13 | Canadian Pharmacists Association. | ||||
| 14 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 15 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 16 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 17 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 18 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 19 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 20 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 21 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 22 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 23 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 24 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
