Details of the Drug
General Information of Drug (ID: DM264B3)
| Drug Name |
Dimenhydrinate
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Gravamin (TN); Gravol (TN); Vertirosan (TN); Dimenhydrinate (JP15/USP/INN); 2-(diphenylmethoxy)-N,N-dimethylethanaminium 8-chloro-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-ide; 2-benzhydryloxyethyl(dimethyl)azanium; 8-chloro-1,3-dimethyl-2-oxopurin-6-olate; 8-chloro-1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione-2-(diphenylmethoxy)-N,N-dimethylethanamine (1:1)
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiemetics
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
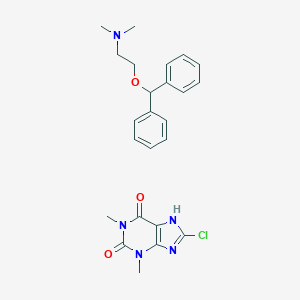 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 470 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||||||
| Rotatable Bond Count | 6 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 5 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Meniere disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | AB31.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Dimenhydrinate
Coadministration of a Drug Treating the Disease Different from Dimenhydrinate (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Dimenhydrinate FDA Label | ||||
|---|---|---|---|---|---|
| 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040519. | ||||
| 3 | Sandoz Canada: Dimenhydrinate Intramuscular and Intravenous Injection | ||||
| 4 | Dailymed: Dimenhydrinate Intramuscular and Intravenous Injection | ||||
| 5 | Histamine 1 receptor antagonist in symptomatic treatment of renal colic accompanied by nausea: two birds with one stone Urology. 2009 Jan;73(1):32-6. | ||||
| 6 | Cholinesterase inhibition by phenothiazine and nonphenothiazine antihistaminics: analysis of its postulated role in synergizing organophosphate toxicity. Toxicol Appl Pharmacol. 1975 Feb;31(2):179-90. | ||||
| 7 | Cohen MA, Alfonso CA, Mosquera M. Development of urinary retention during treatment with clozapine and meclizine [published correction appears in Am J Psychiatry 1994 Jun;151(6):952]. Am J Psychiatry. 1994;151(4):619-620. [PMID: 8147469] | ||||
| 8 | Product Information. Motilium (domperidone). Janssen-Ortho Inc, Toronto, ON. | ||||
| 9 | Benjamin KW "Toxicity of ocular medications." Int Ophthalmol Clin 19 (1979): 199-255. [PMID: 376469] | ||||
| 10 | Kulik AV, Wilbur R "Delirium and stereotypy from anticholinergic antiparkinson drugs." Prog Neuropsychopharmacol Biol Psychiatry 6 (1982): 75-82. [PMID: 7202232] | ||||
| 11 | Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC "Explicit criteria for determining inappropriate medication use in nursing home residents." Arch Intern Med 151 (1991): 1825-32. [PMID: 1888249] | ||||
| 12 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 13 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 14 | Cole JM, Sheehan AH, Jordan JK "Concomitant use of ipratropium and tiotropium in chronic obstructive plmonary disease." Ann Pharmacother 46 (2012): 1717-21. [PMID: 23170031] | ||||
| 15 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 16 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 17 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 18 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
