Details of the Drug
General Information of Drug (ID: DM5URA2)
| Drug Name |
Chlorpheniramine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Allergican; Allergisan; Antagonate; Chloropheniramine; Chlorophenylpyridamin; Chlorophenylpyridamine; Chloropiril; Chloroprophenpyridamine; Chlorphenamine; Chlorphenaminum; Chlorpheniraminum; Chlorprophenpyridamine; Clofeniramina; Clorfenamina; Clorfeniramina; Cloropiril; Haynon; Hayon; Histadur; ISOCLOR; Kloromin; Phenetron; PiriIton; Piriton; Polaronil; Telachlor; Teldrin; Chlorphenamine [INN]; Clorfeniramina [Italian]; Pediacare Allergy Formula; [3H]Chlorpheniramine; Aller-Chlor; Chlo-amine; Chlor-Pro; Chlor-Trimeton Repetabs; Chlor-trimeton; Chlorphenamine (INN); Chlorphenaminum [INN-Latin]; Clofeniramina (TN); Clorfenamina [INN-Spanish]; Comakin (TN); Gen-Allerate; Novo-Pheniram; Piriton (TN); Chlor-Trimeton (TN); Chlor-Tripolon (TN); CHLORPHENIRAMINE (SEE ALSO: CHLORPHENIRAMINE MALEATE (CAS113-92-8)); Gamma-(4-Chlorophenyl)-gamma-(2-pyridyl)propyldimethylamine; Gamma-(4-Chlorophenyl)-N,N-dimethyl-2-pyridinepropanamine; 1-(p-Chlorophenyl)-1-(2-pyridyl)-3-N,N-dimethylpropylamine; 1-(p-Chlorophenyl)-1-(2-pyridyl)-3-dimethylaminopropane; 2-(p-Chloro-alpha-(2-(dimethylamino)ethyl)benzyl)pyridine; 3-(4-chlorophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine; 3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine; 3-(p-Chlorophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine; 4-Chloropheniramine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiallergic Agents
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
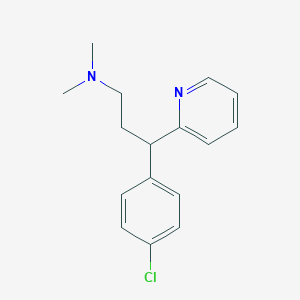 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 274.79 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Chlorpheniramine
Coadministration of a Drug Treating the Disease Different from Chlorpheniramine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6976). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Intact cell binding for in vitro prediction of sedative and non-sedative histamine H1-receptor antagonists based on receptor internalization. J Pharmacol Sci. 2008 May;107(1):66-79. | ||||
| 6 | Carrier mediated transport of chlorpheniramine and chlorcyclizine across bovine olfactory mucosa: implications on nose-to-brain transport. J Pharm Sci. 2005 Mar;94(3):613-24. | ||||
| 7 | Drug Interactions Flockhart Table | ||||
| 8 | The roles of CYP2D6 and stereoselectivity in the clinical pharmacokinetics of chlorpheniramine. Br J Clin Pharmacol. 2002 May;53(5):519-25. | ||||
| 9 | Cholinesterase inhibition by phenothiazine and nonphenothiazine antihistaminics: analysis of its postulated role in synergizing organophosphate toxicity. Toxicol Appl Pharmacol. 1975 Feb;31(2):179-90. | ||||
| 10 | Impact of CYP2D6*10 on H1-antihistamine-induced hypersomnia. Eur J Clin Pharmacol. 2006 Dec;62(12):995-1001. doi: 10.1007/s00228-006-0210-3. Epub 2006 Nov 7. | ||||
| 11 | A Gene Expression Biomarker Predicts Heat Shock Factor 1 Activation in a Gene Expression Compendium. Chem Res Toxicol. 2021 Jul 19;34(7):1721-1737. doi: 10.1021/acs.chemrestox.0c00510. Epub 2021 Jun 25. | ||||
| 12 | Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4. | ||||
| 13 | Block of HERG k channel by classic histamine h(1) receptor antagonist chlorpheniramine. Korean J Physiol Pharmacol. 2009 Jun;13(3):215-20. doi: 10.4196/kjpp.2009.13.3.215. Epub 2009 Jun 30. | ||||
| 14 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 15 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 16 | Kulik AV, Wilbur R "Delirium and stereotypy from anticholinergic antiparkinson drugs." Prog Neuropsychopharmacol Biol Psychiatry 6 (1982): 75-82. [PMID: 7202232] | ||||
| 17 | Cohen MA, Alfonso CA, Mosquera M. Development of urinary retention during treatment with clozapine and meclizine [published correction appears in Am J Psychiatry 1994 Jun;151(6):952]. Am J Psychiatry. 1994;151(4):619-620. [PMID: 8147469] | ||||
| 18 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 19 | Cole JM, Sheehan AH, Jordan JK "Concomitant use of ipratropium and tiotropium in chronic obstructive plmonary disease." Ann Pharmacother 46 (2012): 1717-21. [PMID: 23170031] | ||||
| 20 | Canadian Pharmacists Association. | ||||
| 21 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 22 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 23 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 24 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 25 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 26 | Product Information. Vizimpro (dacomitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 27 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 28 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 29 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 30 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 31 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 32 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 33 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 35 | Dufresne RL, Weber SS, Becker RE "Bupropion hydrochloride." Drug Intell Clin Pharm 18 (1984): 957-64. [PMID: 6439541] | ||||
| 36 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 37 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
