Details of the Drug
General Information of Drug (ID: DMFA5MY)
| Drug Name |
Metoclopramide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cerucal; Clopra; Clopromate; Duraclamid; Elieten; Emetid; Emitasol; Emperal; Eucil; Gastrese; Gastrobid; Gastromax; Gastronerton; Gastrosil; Gastrotablinen; Gastrotem; Imperan; Maxeran; Maxolon; Meclopran; Metaclopramide; Metaclopromide; Metamide; Methochlopramide; Methoclopramide; Metochlopramide; Metoclol; Metoclopramida; Metoclopramidum; Metocobil; Metramid; Moriperan; Mygdalon; Octamide; Parmid; Paspertin; Peraprin; Plasil; Pramidin; Pramiel; Pramin; Primperan; Reclomide; Reglan; Reliveran; Terperan; Metoclopramide Omega; DEL 1267; Apo-Metoclop; CLOPRA-YELLOW; Clopra-Yellow; Degan (TN); Elieten (TN); Gastro-Timelets; Maxeran (TN); Maxolon (TN); Metoclopramida [INN-Spanish]; Metoclopramide Monohydrochloride, Monohydrate; Metoclopramidum [INN-Latin]; Neu-Sensamide; Nu-Metoclopramide; Plasil (pharmaceutical); Pms-Metoclopramide; Primperan (TN); Pylomid (TN); Reglan (TN); Terperan (TN); Metoclopramide (JP15/INN); Metoclopramide [INN:BAN:JAN]; N-(Diethylaminoethyl)-2-methoxy-4-amino-5-chlorobenzamide; O-Anisamide, 4-amino-5-chloro-N-(2-(diethylamino)ethyl)-(8CI); Benzamide, 4-amino-5-chloro-N-(2-(diethylamino)ethyl)-2-methoxy-(9CI); (metaclopramide)4-Amino-5-chloro-N-(2-diethylamino-ethyl)-2-methoxy-benzamide; (metoclopramide)4-Amino-5-chloro-N-(2-diethylamino-ethyl)-2-methoxy-benzamide; 1,5-Dimethyl-2-phenyl-1,2-dihydro-pyrazol-3-one; 2-Methoxy-4-amino-5-chloro-N,N-dimethylaminoethyl)benzamide; 2-Methoxy-5-chloroprocainamide; 2-methoxy-4-amino-5-chloro-N,N-(dimethylaminoethyl)benzamide; 4 Amino-5-chloro-N-(2-(diethylamino)ethyl)-2-methoxybenzamide; 4-Amino-5-chloro-2-methoxy-N-(beta-diethylaminoethyl)benzamide; 4-Amino-5-chloro-N-(2-(diethylamino)ethyl)-2-methoxybenzamide; 4-Amino-5-chloro-N-(2-(diethylamino)ethyl)-o-anisamide; 4-Amino-5-chloro-N-(2-diethylamino-ethyl)-2-methoxy-benzamide; 4-Amino-5-chloro-N-(2-diethylamino-ethyl)-2-methoxy-benzamide (Mcp); 4-Amino-5-chloro-N-(2-diethylamino-ethyl)-2-methoxy-benzamide (metoclopramide); 4-Amino-5-chloro-N-(2-diethylamino-ethyl)-2-methoxy-benzamide(Metoclopramide); 4-amino-5-chloro-N-(2-diethylaminoethyl)-2-methoxybenzamide; 4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxybenzamide; 5-Chloro-2-methoxyprocainamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiemetics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
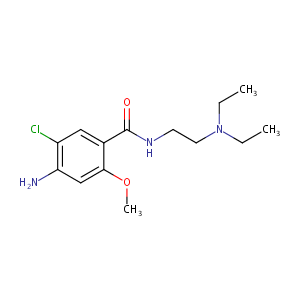 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 299.79 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Nausea | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | MD90 | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Metoclopramide
Coadministration of a Drug Treating the Disease Different from Metoclopramide (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 241). | ||||
|---|---|---|---|---|---|
| 2 | Clinical pharmacokinetics of metoclopramide. Clin Pharmacokinet. 1983 Nov-Dec;8(6):523-9. doi: 10.2165/00003088-198308060-00003. | ||||
| 3 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 4 | Metoclopramide FDA label | ||||
| 5 | Jiang G, Zhang BB: Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003 Apr;284(4):E671-8. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | Ross-Lee LM, Eadie MJ, Hooper WD, Bochner F: Single-dose pharmacokinetics of metoclopramide. Eur J Clin Pharmacol. 1981;20(6):465-71. doi: 10.1007/bf00542101. | ||||
| 9 | Mechanisms for metoclopramide-mediated sensitization and haloperidol-induced catalepsy in rats. Eur J Pharmacol. 2008 Jun 10;587(1-3):181-6. | ||||
| 10 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | ||||
| 11 | Metoclopramide is metabolized by CYP2D6 and is a reversible inhibitor, but not inactivator, of CYP2D6. Xenobiotica. 2014 Apr;44(4):309-319. | ||||
| 12 | Interactions of metoclopramide and ergotamine with human 5-HT(3A) receptors and human 5-HT reuptake carriers. Br J Pharmacol. 2005 Oct;146(4):543-52. doi: 10.1038/sj.bjp.0706351. | ||||
| 13 | Clinical response and side effects of metoclopramide: associations with clinical, demographic, and pharmacogenetic parameters. J Clin Gastroenterol. 2012 Jul;46(6):494-503. doi: 10.1097/MCG.0b013e3182522624. | ||||
| 14 | Association of ABCB1, 5-HT3B receptor and CYP2D6 genetic polymorphisms with ondansetron and metoclopramide antiemetic response in Indonesian cancer patients treated with highly emetogenic chemotherapy. Jpn J Clin Oncol. 2011 Oct;41(10):1168-76. doi: 10.1093/jjco/hyr117. Epub 2011 Aug 11. | ||||
| 15 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 16 | Effects of metoclopramide on duodenal motility and flow events, glucose absorption, and incretin hormone release in response to intraduodenal glucose infusion. Am J Physiol Gastrointest Liver Physiol. 2010 Dec;299(6):G1326-33. doi: 10.1152/ajpgi.00476.2009. Epub 2010 Sep 9. | ||||
| 17 | Effect of drugs interacting with the dopaminergic receptors on glucose levels and insulin release in healthy and type 2 diabetic subjects. Am J Ther. 2008 Jul-Aug;15(4):397-402. doi: 10.1097/MJT.0b013e318160c353. | ||||
| 18 | Differences in the opioid control of luteinizing hormone secretion between pathological and iatrogenic hyperprolactinemic states. J Clin Endocrinol Metab. 1987 Mar;64(3):508-12. doi: 10.1210/jcem-64-3-508. | ||||
| 19 | Comparison of the effects of metoclopramide and domperidone on HERG channels. Pharmacology. 2005 Apr;74(1):31-6. doi: 10.1159/000083234. Epub 2005 Jan 7. | ||||
| 20 | Growth hormone and prolactin secretion after metoclopramide administration (DA2 receptor blockade) in fertile women. Horm Metab Res. 2001 Sep;33(9):536-9. doi: 10.1055/s-2001-17214. | ||||
| 21 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 22 | Filibeck DJ, Grimm D, Forman WB, Leidner BA "Metoclopramide-induced hypertensive crisis." Clin Pharm 3 (1984): 548-9. [PMID: 6541544] | ||||
| 23 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 24 | Product Information. Noxafil (posaconazole). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 25 | Bateman DN, Rawlins MD, Simpson JM "Extrapyramidal reactions with metoclopramide." Br Med J (Clin Res Ed) 291 (1985): 930-2. [PMID: 3929968] | ||||
| 26 | Manara AR, Shelly MP, Quinn K, Park GR "The effect of metoclopramide on the absorption of oral controlled release morphine." Br J Clin Pharmacol 25 (1988): 518-21. [PMID: 3382595] | ||||
| 27 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 28 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 29 | Product Information. Motilium (domperidone). Janssen-Ortho Inc, Toronto, ON. | ||||
| 30 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 31 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 32 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 33 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 34 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 35 | Product Information. Vizimpro (dacomitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 36 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 37 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 38 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 39 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 40 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 41 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 42 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 43 | Mims RB, Scott CL, Modebe O, Bethune JE "Inhibition of L-dopa-induced growth hormone stimulation by pyridoxine and chlorpromazine." J Clin Endocrinol Metab 40 (1975): 256-9. [PMID: 1117978] | ||||
| 44 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
| 45 | Ganzini L, Casey DE, Hoffman WF, McCall AL "The prevalence of metoclopramide-induced tardive dyskinesia and acute extrapyramidal movement disorders." Arch Intern Med 153 (1993): 1469-75. [PMID: 8512437] | ||||
