Details of the Drug
General Information of Drug (ID: DMN3UXQ)
| Drug Name |
Diethylstilbestrol
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acnestrol; Agostilben; Antigestil; Apstil; Bufon; Climaterine; Comestrol; Cyren; DES; Desma; Destrol; Diaethylstilboestrolum; Diastyl; Dibestrol; Dicorvin; Diethylstilbesterol; Diethylstilbestrolum; Diethylstilboesterol; Dietilestilbestrol; Dietilstilbestrolo; Distilbene; Domestrol; Dyestrol; Estril; Estrobene; Estrogenine; Estromenin; Estrosyn; Fonatol; Grafestrol; Gynopharm; Hibestrol; Idroestril; Iscovesco; Makarol; Menostilbeen; Micrest; Microest; Milestrol; OeKolp; Oestrogenine; Oestrolmensil; Oestromenin; Oestromensil; Oestromensyl; Oestromienin; Oestromon; Pabestrol; Palestrol; Protectona; Sedestran; Serral; Sexocretin; Sibol; Sintestrol; Stibilium; Stilbestroform; Stilbestrol; Stilbestrone; Stilbetin; Stilboefral; Stilboestroform; Stilboestrol; Stilbofolin; Stilbofollin; Stilbol; Stilkap; Strobene; Synestrin; Synthestrin; Synthoestrin; Synthofolin; Syntofolin; Tampovagan; Tylosterone; Vagestrol; APS Brand of Diethylstilbestrol; Anti gestil; Co Pharma Brand of Diethylstilbestrol; Comestrol estrobene; Cyren A; Diethylstilbestrol BP; Dietilestilbestrol [Spanish]; Dietilstilbestrolo [DCIT]; Estilbin MCO; Gerda Brand of Diethylstilbestrol; Oestrol vetag; Percutatrine oestrogenique iscovesco; ST IL; Stilbene Estrogen; Tampovagan stilboestrol; DiBestrol 2 Premix; MG 137; Rumestrol 1; Rumestrol 2; Cis-Des; Cis-Diethylstilbesterol; Cis-Diethylstilbestrol; Co-Pharma Brand of Diethylstilbestrol; DES (synthetic estrogen); Dawe's destrol; Di-Estryl; Diethylstilbestrol (DES); Diethylstilbestrol [USAN:INN]; Diethylstilbestrol, Disodium Salt; Diethylstilbestrolum [INN-Latin]; Dietilestilbestrol [INN-Spanish]; E-Diethylstilbestrol; Estilbin (MCO); Estrogen, Stilbene; Hi-Bestrol; Neo-Oestranol 1; Neo-Oestranol I; Neo-oe stranol 1; New-Estranol 1; Stil-Rol; Stilbestrol (TN); TRANS-DIETHYSTILBESTEROL; TRANSGENIC MODEL EVALUATION (DES); Trans-Diethylstilbesterol; Trans-Diethylstilbestrol; Trans-Diethylstilboesterol; DiBestrol "2" Premix; Dibestrol '2' premix; Diethylstilbestrol (USP/INN); Alpha,alpha'-Diethylstilbenediol; Diethylstilbestrol, (Z)-Isomer; Alpha,alpha'-Diethyl-4,4'-stilbenediol; (E)-3,4-Bis(4-hydroxyphenyl)-3-hexene; (E)-4,4'-(hex-3-ene-3,4-diyl)diphenol; (E)-Diethylstilbestrol; 3,4'(4,4'-Dihydroxyphenyl)hex-3-ene; 3,4-Bis(p-hydroxyphenyl)-3-hexene; 3,4-bis(4-hydroxyphenyl)hex-3-ene; 4,4'-(3E)-hex-3-ene-3,4-diyldiphenol; 4,4'-Dihydroxydiethylstilbene; 4,4'-hex-3-ene-3,4-diyldiphenol
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||
| Structure |
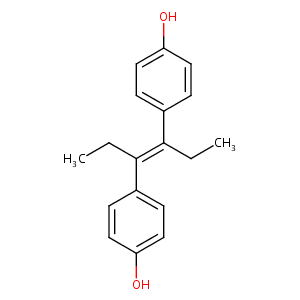 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 268.3 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.1 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||
| ADMET Property | ||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Gonorrheal vaginitis | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | GA02 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Diethylstilbestrol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
