Details of the Drug
General Information of Drug (ID: DMKWFBT)
| Drug Name |
Melatonin
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
73-31-4; Melatonine; N-Acetyl-5-methoxytryptamine; Circadin; 5-Methoxy-N-acetyltryptamine; N-[2-(5-Methoxy-1H-indol-3-yl)ethyl]acetamide; N-(2-(5-Methoxy-1H-indol-3-yl)ethyl)acetamide; Melatol; Melovine; Melatonex; N-[2-(5-methoxyindol-3-yl)ethyl]acetamide; Acetamide, N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-; UNII-JL5DK93RCL; N-(2-(5-Methoxyindol-3-yl)ethyl)acetamide; N-acetyl-5-methoxy-tryptamine; NSC 113928; CCRIS 3472; CHEMBL45; EINECS 200-797-7; JL5DK93RCL; Acetamide, N-(2-(5-methoxyindol-3-yl)ethyl)-; BRN 0205542; Circadin; Melapure; Melatonina; Posidorm; Vivitas; Night Rest; Pineal Hormone; Revital Melatonin; Rx Balance; Sleep Right; IN1244; M 5250; M1105; ML1; MT6; TNP00300; M-1200; M-1250; Mela-T; Melatonex, Melatonin; Melatonina (TN); NMR/14327425; Nature'S Harmony; PREVENTION 2 (MELATONIN); PREVENTION 3 (MELATONIN); PREVENTION 4 (MELATONIN); PREVENTION 5 (MELATONIN); PREVENTION 1 (MELATONIN) (PREVENTION 1); Acetamide, {N-[2-(5-methoxyindol-3-yl)ethyl]-}; Acetamide, N-[2-(5-methoxyindol-3-yl)ethyl]-(6CI,8CI); N-[2-(5-Methoxy-1H-indol-3-yl)-ethyl]-acetamide; Acetamide, N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-(9CI); Acetamide, {N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-}; Acetamide, N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-(9CI); 4-ACETAMIDO-4'-ISOTHIO-CYANATOSTILBENE-2,2'-DISULFONIC ACID; [3H]melatonin; N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Central Nervous System Stimulants
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
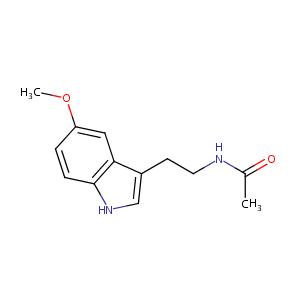 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 232.28 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Melatonin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
