Details of the Drug
General Information of Drug (ID: DMMFCIH)
| Drug Name |
Clotrimazole
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Canesten; Canestene; Canestine; Canifug; Chlotrimazole; Cimitidine; Clomatin; Clotrimaderm; Clotrimazol; Clotrimazolum; Clotrimeizol; Cutistad; Empecid; Esparol; FemCare; Gynix; Kanesten; Klotrimazole; Lotrimax; Lotrimin; Monobaycuten; Mycelax; Mycelex; Mycofug; Mycosporin; Mykosporin; Nalbix; Pedisafe; Rimazole; Stiemazol; Tibatin; Trimysten; Canesten Cream; Canesten Solution; Clotrimazole Schering Brand; Desamix F; Fem Care; Gyne lotrimin; Lotrimin AF Cream; Lotrimin AF Lotion; Lotrimin AF Solution; Lotrimin Af; Lotrimin Cream; Lotrimin Lotion; Lotrimin Solution; Mycelex Cream; Mycelex G; Mycelex OTC; Mycelex Solution; Mycelex Troches; Mycelex Twin Pack; Myclo Cream; Myclo Solution; Myclo Spray Solution; Schering Brand of Clotrimazole; B 5097; Bay b 9057; Bayer Brand 1 of Clotrimazole; Bayer Brand 2 of Clotrimazole; C 6019; FB 5097; FB b 5097; Mycelex 7; Trivagizole 3; Bay-B 5097; Candid Vaginal (TN); Candinil (TN); Canesten (TN); Canesten 1-Day Therapy; Canesten 3-Day Therapy; Canesten 6-Day Therapy; Clobrate VT (TN); Clotrimazol [INN-Spanish]; Clotrimazolum [INN-Latin]; DRG-0072; Gino-Lotremine; Gyne-Lotrimin; Gyne-Lotrimin 3; Gyne-Lotrimin 3 Combination Pack; Gyne-Lotrimin Combination Pack; Lotrimin (TN); Lotrimin AF Jock-Itch Cream; Mono-baycuten; Mycelex (TN); Mycelex-7; Mycelex-7 Combination Pack; Mycelex-G; Mycelex: MycosporinRimazole; Myclo-Gyne; Neo-Zol Cream; Pan-Fungex; Cancap-VT (TN); Candid - V Gel (TN); Canesten 1-Day Cream Combi-Pak; Canesten Combi-Pak 1-Day Therapy; Canesten Combi-Pak 3-Day Therapy; Clotrimazole (JP15/USP/INN); Clotrimazole [USAN:INN:BAN:JAN]; (Chlorotrityl)imidazole; 1-(o-Chlorotrityl)imidazole
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Yeast and other fungi
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
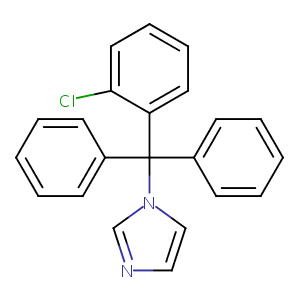 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 344.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Cutaneous candidiasis | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1F23.14 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Clotrimazole (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Clotrimazole FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2330). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Clotrimazole Cream, DailyMed | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Mode of action of clotrimazole: implications for therapy. Am J Obstet Gynecol. 1985 Aug 1;152(7 Pt 2):939-44. | ||||
| 7 | Tacrolimus interaction with clotrimazole: a concise case report and literature review. P T. 2010 Oct;35(10):568-9. | ||||
| 8 | Direct stimulation of adenylyl cyclase 9 by the fungicide imidazole miconazole. Naunyn Schmiedebergs Arch Pharmacol. 2019 Apr;392(4):497-504. doi: 10.1007/s00210-018-01610-1. Epub 2019 Jan 3. | ||||
| 9 | Comparative assessment of the inhibition of recombinant human CYP19 (aromatase) by azoles used in agriculture and as drugs for humans. Endocr Res. 2004 Aug;30(3):387-94. | ||||
| 10 | Improved assays for xenosensor activation based on reverse transfection. Toxicol In Vitro. 2015 Oct;29(7):1759-65. doi: 10.1016/j.tiv.2015.07.011. Epub 2015 Jul 14. | ||||
| 11 | Influence of redox-active compounds and PXR-activators on human MRP1 and MRP2 gene expression. Toxicology. 2002 Feb 28;171(2-3):137-46. | ||||
| 12 | Activation of p38 Mitogen-Activated Protein Kinase by Clotrimazole Induces Multidrug Resistance-Associated Protein 3 Activation through a Novel Transcriptional Element. J Pharmacol Exp Ther. 2016 Oct;359(1):102-9. doi: 10.1124/jpet.115.231589. Epub 2016 Aug 9. | ||||
| 13 | Combination of imatinib and clotrimazole enhances cell growth inhibition in T47D breast cancer cells. Chem Biol Interact. 2015 May 25;233:147-56. doi: 10.1016/j.cbi.2015.03.028. Epub 2015 Apr 8. | ||||
| 14 | Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996 Feb;49(2):311-8. | ||||
| 15 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | ||||
| 16 | Effects of clotrimazole on the growth, morphological characteristics, and cisplatin sensitivity of human glioblastoma cells in vitro. J Neurosurg. 1999 May;90(5):918-27. doi: 10.3171/jns.1999.90.5.0918. | ||||
| 17 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 18 | Caballero-Granado FJ, Viciana P, Cordero E, Gomez-Vera MJ, del Nozal M, Lopez-Cortes LF "Ergotism related to concurrent administration of ergotamine tartrate and ritonavir in an AIDS patient." Antimicrob Agents Chemother 41 (1997): 1207. [PMID: 9145904] | ||||
| 19 | Bidstrup TB, Bjornsdottir I, Sidelmann UG, Thomsen MS, Hansen KT "CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide." Br J Clin Pharmacol 56 (2003): 305-14. [PMID: 12919179] | ||||
| 20 | Product Information. Aubagio (teriflunomide). Genzyme Corporation, Cambridge, MA. | ||||
| 21 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 22 | Ament PW, Paterson A "Drug interactions with the nonsedating antihistamines." Am Fam Physician 56 (1997): 223. [PMID: 9225677] | ||||
| 23 | Product Information. Aricept (donepezil). Pfizer US Pharmaceuticals, New York, NY. | ||||
| 24 | Heinig R, Adelmann HG, Ahr G "The effect of ketoconazole on the pharmacokinetics, pharmacodynamics and safety of nisoldipine." Eur J Clin Pharmacol 55 (1999): 57-60. [PMID: 10206086] | ||||
| 25 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 26 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 27 | Ahonen J, Olkkola KT, Neuvonen PJ "Effect of itraconazole and terbinafine on the pharmacokinetics and pharmacodynamics of midazolam in healthy volunteers." Br J Clin Pharmacol 40 (1995): 270-2. [PMID: 8527290] | ||||
| 28 | Hedaya MA, El-Afify DR, El-Maghraby GM "The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers." Biopharm Drug Dispos 27 (2006): 103-10. [PMID: 16372380] | ||||
| 29 | Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN "Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole." Ann Pharmacother 38 (2004): 46-9. [PMID: 14742792] | ||||
| 30 | Product Information. Elidel (pimecrolimus topical). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 31 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 32 | Product Information. Ixempra (ixabepilone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 33 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Abbas R, Hug BA, Leister C, Burns J, Sonnichsen D "Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects." Br J Clin Pharmacol 71 (2011): 522-7. [PMID: 21395644] | ||||
| 35 | Product Information. Verzenio (abemaciclib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 36 | Devenport MH, Crook D, Wynn V, Lees LJ "Metabolic effects of low-dose fluconazole in healthy female users and non-users of oral contraceptives." Br J Clin Pharmacol 27 (1989): 851-9. [PMID: 2547410] | ||||
| 37 | Product Information. Jevtana (cabazitaxel). sanofi-aventis , Bridgewater, NJ. | ||||
| 38 | Product Information. Fareston (toremifene). Schering Laboratories, Kenilworth, NJ. | ||||
| 39 | Product Information. Opsumit (macitentan). Actelion Pharmaceuticals US Inc, South San Francisco, CA. | ||||
| 40 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 41 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 42 | Ekstrom G, Gunnarsson UB "Ropivacaine, a new amide-type local anesthetic agent, is metabolized by cytochromes P450 1A and 3A in human liver microsomes." Drug Metab Dispos 24 (1996): 955-61. [PMID: 8886604] | ||||
| 43 | Bartkowski RR, Goldberg ME, Larijani GE, Boerner T "Inhibition of alfentanil metabolism by erythromycin." Clin Pharmacol Ther 46 (1989): 99-102. [PMID: 2501060] | ||||
| 44 | Cobb MN, Desai J, Brown LS Jr, Zannikos PN, Rainey PM "The effect of fluconazole on the clinical pharmacokinetics of methadone." Clin Pharmacol Ther 63 (1998): 655-62. [PMID: 9663180] | ||||
| 45 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 46 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 47 | Product Information. Viibryd (vilazodone). Trovis Pharmaceuticals LLC, New Haven, CT. | ||||
| 48 | Product Information. Celexa (citalopram). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 49 | Product Information. Rexulti (brexpiprazole). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 50 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 51 | Lazar JD, Wilner KD "Drug interactions with fluconazole." Rev Infect Dis 12 Suppl 3 (1990): s327-33. [PMID: 2330488] | ||||
| 52 | Product Information. Ingrezza (valbenazine). Neurocrine Biosciences, Inc., San Diego, CA. | ||||
| 53 | Ahonen J, Olkkola KT, Neuvonen PJ "The effect of the antimycotic itraconazole on the pharmacokinetics and pharmacodynamics of diazepam." Fundam Clin Pharmacol 10 (1996): 314-8. [PMID: 8836707] | ||||
| 54 | Nakasa H, Nakamura H, Ono S, Tsutsui M, Kiuchi M, Ohmori S, Kitada M "Prediction of drug-drug interactions of zonisamide metabolism in humans from in vitro data." Eur J Clin Pharmacol 54 (1998): 177-83. [PMID: 9626925] | ||||
| 55 | Product Information. Aliqopa (copanlisib). Bayer Pharmaceutical Inc, West Haven, CT. | ||||
| 56 | Product Information. VESIcare (solifenacin). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 57 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 58 | Brynne N, Forslund C, Hallen B, Gustafsson LL, Bertilsson L "Ketoconazole inhibits the metabolism of tolterodine in subjects with deficient CYP2D6 activity." Br J Clin Pharmacol 48 (1999): 564-72. [PMID: 10583027] | ||||
| 59 | Product Information. Enablex (darifenacin). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 60 | Bedford TA, Rowbotham DJ "Cisapride: drug interactions of clinical significance." Drug Saf 15 (1996): 167-75. [PMID: 8879971] | ||||
| 61 | Ausband SC, Goodman PE "An unusual case of clarithromycin associated ergotism." J Emerg Med 4 (2001): 411-3. [PMID: 11728770] | ||||
| 62 | Product Information. Inspra (eplerenone). Searle, Chicago, IL. | ||||
| 63 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 64 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 65 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 66 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 67 | Elsharkawy AM, Schwab U, McCarron B, et al. "Efavirenz induced acute liver failure requiring liver transplantation in a slow drug metaboliser." J Clin Virol 58 (2013): 331-3. [PMID: 23763943] | ||||
| 68 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 69 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 70 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 71 | Product Information. Reyataz (atazanavir). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 72 | Product Information. Selzentry (maraviroc). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 73 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 74 | Canadian Pharmacists Association. | ||||
| 75 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 76 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 77 | Alderman CP, Gebauer MG, Gilbert AL, Condon JT "Possible interaction of zopiclone and nefazodone." Ann Pharmacother 35 (2001): 1378-80. [PMID: 11724087] | ||||
| 78 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 79 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 80 | Farkas D, Volak L, Harmatz J, von Moltke L, Court M, Greenblatt D "Short-term clarithromycin administration impairs clearance and enhances pharmacodynamic effects of trazodone but not of zolpidem." Clin Pharmacol Ther 85 (2009): 644-50. [PMID: 19242403] | ||||
| 81 | Product Information. Xalkori (crizotinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 82 | Product Information. Zykadia (ceritinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 83 | Product Information. Zepzelca (lurbinectedin). Jazz Pharmaceuticals, Palo Alto, CA. | ||||
| 84 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 85 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 86 | Product Information. Gavreto (pralsetinib). Blueprint Medicines Corporation, Cambridge, MA. | ||||
| 87 | Abernethy DR, Wesche DL, Barbey JT, et al. "Stereoselective halofantrine disposition and effect: concentration-related QTc prolongation." Br J Clin Pharmacol 51 (2001): 231-7. [PMID: 11298069] | ||||
| 88 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 89 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 90 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 91 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 92 | Product Information. Clolar (clofarabine). sanofi-aventis, Bridgewater, NJ. | ||||
| 93 | Product Information. Imbruvica (ibrutinib). Pharmacyclics Inc, Sunnyvale, CA. | ||||
| 94 | Product Information. Iclusig (ponatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 95 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 96 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 97 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 98 | Hamberg P, Woo MM, Chen LC, et al. "Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor." Cancer Chemother Pharmacol 68 (2011): 805-13. [PMID: 21706316] | ||||
| 99 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 100 | Product Information. Gleevec (imatinib mesylate). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 101 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 102 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 103 | Product Information. Meridia (sibutramine). Knoll Pharmaceutical Company, Whippany, NJ. | ||||
| 104 | Product Information. Orlaam (levomethadyl acetate) Roxanne Laboratories Inc, Columbus, OH. | ||||
| 105 | Product Information. Bextra (valdecoxib). Pharmacia Corporation, Peapack, NJ. | ||||
| 106 | Product Information. Lynparza (olaparib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 107 | Product Information. Butorphanol Tartrate (butorphanol). Apotex Corporation, Weston, FL. | ||||
| 108 | Product Information. Buprenex (buprenorphine). Reckitt and Colman Pharmaceutical, Richmond, VA. | ||||
| 109 | Hutchinson MR, Menelaou A, Foster DJ, Coller JK, Somogyi AA "CYP2D6 and CYP3A4 involvement in the primary oxidative metabolism of hydrocodone by human liver microsomes." Br J Clin Pharmacol 57 (2004): 287-97. [PMID: 14998425] | ||||
| 110 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 111 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 112 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 113 | Product Information. Xtandi (enzalutamide). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 114 | Franco-Salinas G, de la Rosette JJ, Michel MC "Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations." Clin Pharmacokinet 49 (2010): 177-88. [PMID: 20170206] | ||||
| 115 | Product Information. Rapaflo (silodosin). Watson Pharmaceuticals, Corona, CA. | ||||
| 116 | Product Information. Duagen (dutasteride). GlaxoSmithKline Healthcare, Pittsburgh, PA. | ||||
| 117 | Product Information. Uroxatral (alfuzosin). sanofi-aventis , Bridgewater, NJ. | ||||
| 118 | Product Information. Tracleer (bosentan). Acetelion Pharmaceuticals US, Inc, South San Francisco, CA. | ||||
| 119 | Product Information. Afinitor (everolimus). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 120 | Product Information. Torisel (temsirolimus). Wyeth-Ayerst Laboratories, Philadelphia, PA. | ||||
| 121 | DeVane CL, Nemeroff CB "Clinical pharmacokinetics of quetiapine - An atypical antipsychotic." Clin Pharmacokinet 40 (2001): 509-22. [PMID: 11510628] | ||||
| 122 | Product Information. Abilify (aripiprazole). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 123 | Dresser GK, Spence JD, Bailey DG "Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition." Clin Pharmacokinet 38 (2000): 41-57. [PMID: 10668858] | ||||
| 124 | Product Information. Cialis (tadalafil). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 125 | Product Information. Levitra (vardenafil). Bayer, West Haven, CT. | ||||
| 126 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 127 | Li J, Zhao M, He P, Hidalgo M, Baker SD "Differential metabolism of gefitinib and erlotinib by human cytochrome p450 enzymes." Clin Cancer Res 13 (2007): 3731-7. [PMID: 17575239] | ||||
| 128 | Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K "Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60 year old Caucasian woman with ovarian carcinoma." Br J Clin Pharmacol (2015):. [PMID: 26446447] | ||||
| 129 | Product Information. ReVia (naltrexone). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 130 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 131 | Product Information. Cometriq (cabozantinib). Exelixis Inc, S San Francisco, CA. | ||||
| 132 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 133 | Product Information. Orilissa (elagolix). AbbVie US LLC, North Chicago, IL. | ||||
| 134 | Product Information. Allegra (fexofenadine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 135 | Product Information. Rythmol SR (propafenone). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 136 | Fabre G, Julian B, Saint-Aubert B, Joyeux H, Berger Y "Evidence for CYP3A-mediated N-deethylation of amiodarone in human liver microsomal fractions." Drug Metab Dispos 21 (1993): 978-85. [PMID: 7905403] | ||||
