Details of the Drug
General Information of Drug (ID: DM0CVXA)
| Drug Name |
Mechlorethamine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Antimit; Carolysine; Caryolysin; Caryolysine; Chlorethazine; Chlormethine; Cloramin; Clormetina; Dichloren; Embechine; Embichin; MBA; Mebichloramine; Mechlorethanamine; Mechloroethamine; Mecloretamina; Mustargen; Mustine; Mutagen; Nitrogranulogen; Thyldiethylamine; Dichlor amine; Me chloroethamine; Mecloretamina [Italian]; Mustine note; Nitrogen mustard; HN2; T 1024; TL 146; Chloramine (the nitrogen mustard); Chlormethine (INN); Chlormethine [INN:BAN]; Chlormethinum [INN-Latin]; Clormetina [INN-Spanish]; ENT-25294; HN-2; IBS-L0033631; Mustargen (TN); N-Lost; N-Methyl lost; Stickstofflost (ebewe); T-1024; Bis(2-chloroethyl)methylamine; Bis(beta-chloroethyl) methylamine; Bis(beta-chloroethyl)methylamine; Di(2-chloroethyl)methylamine; Methylbis(2-chloroethyl)amine; Methylbis(beta-chloroethyl)amine; Methyldi(2-chloroethyl)amine; N-Methyl-lost; N-Methyl-lost [German]; Nitrogen mustard (HN-2); Mitoxine (*Hydrochloride*); N,N-Di(chloroethyl)methylamine; N-Methyl-bis-chloraethylamin; N-Methyl-bis-chloraethylamin [German]; Nitol (*Hydrochloride*); Stickstofflost (*Hydrochloride*); N,N-Bis(2-chloroethyl)methylamine; N-Methyl-bis(2-chloroethyl)amine; N-Methyl-bis(beta-chloroethyl)amine; Beta,beta'-Dichlorodiethyl-N-methylamine; N-Methyl-2,2'-dichlorodiethylamine; N,N-Bis(2-chloroethyl)-N-methylamine; 1,5-Dichloro-3-methyl-3-azapentane hydrochloride; 2,2'-Dichloro-N-me; 2,2'-Dichloro-N-methyldiethylamine; 2,2'-Dichlorodiethyl-methylamine; 2-Chloro-N-(2-chloroethyl)-N-methylethanamine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
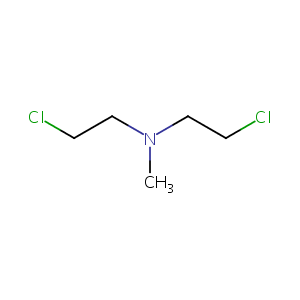 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 156.05 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Mechlorethamine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
