Details of the Drug
General Information of Drug (ID: DM70IK5)
| Drug Name |
Marinol
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dronabinol; TETRAHYDROCANNABINOL; delta9-Tetrahydrocannabinol; delta9-THC; Deltanyne; Dronabinolum; delta-9-tetrahydrocannabinol; Abbott 40566; delta-9-THC; delta(9)-THC; 1972/8/3; delta1-THC; delta(sup 1)-Thc; delta(sup 9)-Thc; 1-trans-delta-9-Tetrahydrocannabinol; THC; Namisol; Dronabinolum [Latin]; 9-tetrahydrocannabinol; delta(9)-Tetrahydrocannabinol; delta(1)-Tetrahydrocannabinol; (-)-delta9-trans-Tetrahydrocannabinol; delta1-Tetrahydrocannabinol; 1-trans-delta9-Tetrahydrocannabinol; delta(9)-Tetrahydrocannibinol; SP; Compassia; Ganja; Hashish; Marincap; MaxEPA; Omegaven; Promega; Relivar; Sonergx; Tetranabinex; Cannabis resin; Drona binol; QCD 84924; SP 104; Tetrahydrocannabinol delta9; CAT-310; DELTA1-THC; DELTA1-Tetrahydrocannabinol;DELTA9-THC; DRG-0138; Delta-THC; Delta1-THC; Delta1-Tetrahydrocannabinol; Delta9-THC; Delta9-Tetrahydrocannabinol; Delta9-Tetrahydrocannabinol solution; Dronabinol [USAN:INN]; Marinol (TN); Pro-Mega;QCD-84924; Trans-tetrahydrocannabinol; DELTA9-trans-Tetrahydrocannabinol; Delta(1)-THC; Delta(1)-Tetrahydrocannabinol; Delta(9)-THC; Delta(9)-Tetrahydrocannabinol; Delta(9)-Tetrahydrocannibinol; Delta(sup 1)-Tetrahydrocannabinol; Delta(sup 1)-Thc; Delta(sup 9)-Tetrahydrocannabinol; Delta(sup 9)-Thc; Delta(sup9)-THC; Delta-9-THC; Delta-9-tetrahydrocannabinol; Delta1-Tetrahydrocannabinol (VAN); Delta9-Tetrahydrocannabinol (VAN); Delta9-trans-Tetrahydrocannabinol; Dronabinol (USP/INN); Omega-3-Fatty acid; THC-delta-9; Trans-delta9-Tetrahydrocannabinol; Cannabinol, tetrahydro-(6CI); Fish oils, n-3 fatty acid-high; Fish oils, omega-3 fatty acid-high; L-delta(sup 1)-tetrahydrocannabinol; L-delta1-trans-Tetrahydrocannabinol; L-trans-delta9-Tetrahydrocannabinol; Trans-delta-9-Tetrahydrocannabinol; Cannabinol, Delta1-tetrahydro-(7CI); Fats and Glyceridic oils, fish, n-3 fatty acid-high; L-delta1-Tetrahydrocannabinol; Trans-DELTA9-Tetrahydrocannabinol; L-delta1-trans-Tetrahydrocannabinol;L-trans-delta9-Tetrahydrocannabinol; Tetrahydrocannabinols (-)-delta1-3,4-trans-form; (-)-DELTA1-Tetrahydrocannabinol; (-)-DELTA9-THC; (-)-DELTA9-Tetrahydrocannabinol; (-)-DELTA9-trans-Tetrahydrocannabinol; (-)-delta(sup9)-trans-Tetrahydrocannabinol; (-)-3,4-trans-Delta1-Tetrahydrocannabinol; (-)-delta(sup 1)-3,4-trans-Tetrahydrocannabinol; (-)-delta1-Tetrahydrocannabinol; (-)-delta9-(trans)-Tetrahydrocannabinol; (-)-delta9-Tetrahydrocannabinol; (-)-trans-DELTA9-Tetrahydrocannabinol; (-)-trans-Delta1-Tetrahydrocannabinol; (-)-trans-Delta9-THC; (-)-trans-delta9-Tetrahydrocannabinol; (L)-delta1-Tetrahydrocannabinol; (l)-delta(sup 1)-Tetrahydrocannabinol; (l)-delta1-Tetrahydrocannabinol; 1-trans-delta(sup9)-tetrahydrocannabinol; 1-trans-D9-Tetrahydrocannabinol; 1-trans-delta(sup 9)-Tetrahydrocannabinol; 14C-DELTA1-Tetrahydrocannabinol; 6H-Dibenzo; 9-delta-Tetrahydrocannabinol; 9-ene-Tetrahydrocannabinol; Delta(9)-tetrahydrocannabinol
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
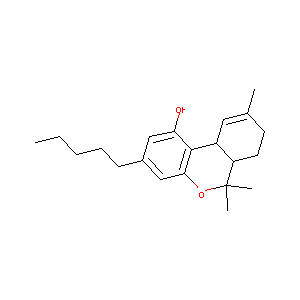 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 314.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Anorexia nervosa cachexia | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6B80 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Marinol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 018651. | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of INSYS Therapeutics. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Ciccolini J, Serdjebi C, Peters GJ, Giovannetti E: Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: an EORTC-PAMM perspective. Cancer Chemother Pharmacol. 2016 Jul;78(1):1-12. doi: 10.1007/s00280-016-3003-0. Epub 2016 Mar 23. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009 Feb;156(3):397-411. | ||||
| 8 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 9 | CYP2C-catalyzed delta9-tetrahydrocannabinol metabolism: kinetics, pharmacogenetics and interaction with phenytoin. Biochem Pharmacol. 2005 Oct 1;70(7):1096-103. | ||||
| 10 | Increasing the intracellular availability of all-trans retinoic acid in neuroblastoma cells. Br J Cancer. 2005 Feb 28;92(4):696-704. | ||||
| 11 | Inhibition of cyclosporine and tetrahydrocannabinol metabolism by cannabidiol in mouse and human microsomes. Xenobiotica. 1996 Mar;26(3):275-84. | ||||
| 12 | Health control over the feeding of troops on the Western and 3d Belorussian Fronts during the Great Patriotic War years. Voen Med Zh. 1975 Jun;(6):86-8. | ||||
| 13 | THC exposure of human iPSC neurons impacts genes associated with neuropsychiatric disorders. Transl Psychiatry. 2018 Apr 25;8(1):89. doi: 10.1038/s41398-018-0137-3. | ||||
| 14 | Gene expression changes in human small airway epithelial cells exposed to Delta9-tetrahydrocannabinol. Toxicol Lett. 2005 Aug 14;158(2):95-107. | ||||
| 15 | Inhibiting Heat Shock Proteins Can Potentiate the Cytotoxic Effect of Cannabidiol in Human Glioma Cells. Anticancer Res. 2015 Nov;35(11):5827-37. | ||||
| 16 | JunD is involved in the antiproliferative effect of Delta9-tetrahydrocannabinol on human breast cancer cells. Oncogene. 2008 Aug 28;27(37):5033-44. | ||||
| 17 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 18 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 19 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 20 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 21 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 22 | Product Information. Trileptal (oxcarbazepine) Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 23 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 24 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 25 | Barry M, Mulcahy F, Merry C, Gibbons S, Back D "Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection." Clin Pharmacokinet 36 (1999): 289-304. [PMID: 10320951] | ||||
| 26 | Product Information. Sustiva (efavirenz). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 27 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 28 | Product Information. Norvir (ritonavir). Abbott Pharmaceutical, Abbott Park, IL. | ||||
| 29 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 30 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 31 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 32 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 33 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 34 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 35 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 36 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 37 | Product Information. Wellbutrin XL (buPROPion). GlaxoSmithKline, Philadelphia, PA. | ||||
| 38 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 39 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 40 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 41 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 42 | Product Information. Artane (trihexyphenidyl). Lederle Laboratories, Wayne, NJ. | ||||
| 43 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 44 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 45 | Product Information. Tracleer (bosentan). Acetelion Pharmaceuticals US, Inc, South San Francisco, CA. | ||||
| 46 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 47 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 48 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
