Details of the Drug
General Information of Drug (ID: DMF70AB)
| Drug Name |
Ginsenoside Rb1
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Arasaponin E1; Ginsenoside rb1; Gynosaponin C; Panax saponin E; Panaxsaponin E; Pseudoginsenoside D; Sanchinoside E1; ginsenoside-Rb1; (20S)-ginsenoside Rb1; 2-O-beta-Glucopyranosyl-(3beta,12beta)-20-((6-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl)oxy)-12-hydroxydammar-24-en-3-yl-beta-D-glucopyranoside; 20(S)-ginsenoside Rb1; 41753-43-9; 7413S0WMH6; BIDD:ER0108; C54H92O23; CHEBI:67989; CHEMBL501515; GRb 1; GS-Rb1; GSRb1; GZYPWOGIYAIIPV-JBDTYSNRSA-N; Gypenoside III; NSC 310103; UNII-7413S0WMH6
|
|||||
| Structure |
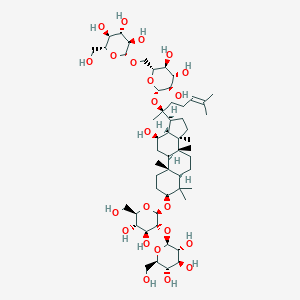 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1109.3 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | |||||
| Rotatable Bond Count (rotbonds) | 16 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 15 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 23 | |||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
