Details of the Drug
General Information of Drug (ID: DMI2QPE)
| Drug Name |
Didanosine
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
didanosine; 69655-05-6; DIDEOXYINOSINE; Videx; Videx EC; Inosine, 2',3'-dideoxy-; ddIno; Didanosina; Didanosinum; DDI; BMY-40900; UNII-K3GDH6OH08; K3GDH6OH08; Didanosinum [INN-Latin]; Didanosina [INN-Spanish]; CHEBI:490877; 9-(2,3-Dideoxy-beta-D-ribofuranosyl)-6-oxopurine; NSC 612049; 9-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,9-dihydro-6H-purin-6-one; 9-[(2R,5R)-5-(HYDROXYMETHYL)TETRAHYDROFURAN-2-YL]-1,9-DIHYDRO-6H-PURIN-6-ONE; NCGC00159514-02; NCGC00090691-03; DRG-0016; BMY 40900; DSSTox_CID_2927
|
|||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
|||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Human Immunodeficiency Virus
|
|||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||
| Structure |
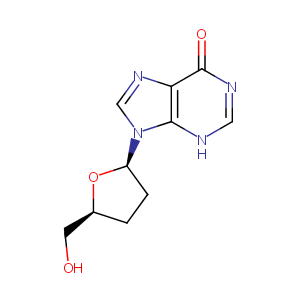 |
|||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 236.23 | ||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.2 | |||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | |||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Didanosine
Coadministration of a Drug Treating the Disease Different from Didanosine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4833). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 6 | The effect of individual antiretroviral drugs on body composition in HIV-infected persons initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009 Jul 1;51(3):298-304. | ||||
| 7 | Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. | ||||
| 8 | Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286. | ||||
| 9 | An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20. | ||||
| 10 | In vitro cytotoxicity and mitochondrial toxicity of tenofovir alone and in combination with other antiretrovirals in human renal proximal tubule cells. Antimicrob Agents Chemother. 2006 Nov;50(11):3824-32. doi: 10.1128/AAC.00437-06. Epub 2006 Aug 28. | ||||
| 11 | Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G910-22. doi: 10.1152/ajpgi.90672.2008. Epub 2009 Jan 22. | ||||
| 12 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 13 | Argov Z, Mastaglia FL "Drug-induced peripheral neuropathies." Br Med J 1 (1979): 663-6. [PMID: 219931] | ||||
| 14 | Canadian Pharmacists Association. | ||||
| 15 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 16 | Honig PK, Gillespie BK "Clinical significance of pharmacokinetic drug interactions with over-the-counter (OTC) drugs." Clin Pharmacokinet 35 (1998): 167-71. [PMID: 9784931] | ||||
| 17 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 18 | Product Information. Adderall (amphetamine-dextroamphetamine) Shire Richwood Pharmaceutical Company, Florence, KY. | ||||
| 19 | Akerele JO, Okhamafe AO "Influence of oral co-administered metallic drugs on ofloxacin pharmacokinetics." J Antimicrob Chemother 28 (1991): 87-94. [PMID: 1663108] | ||||
| 20 | Johnson RD, Dorr MB, Talbot GH, Caille G "Effect of Maalox on the oral absorption of sparfloxacin." Clin Ther 20 (1998): 1149-58. [PMID: 9916608] | ||||
| 21 | Deppermann KM, Lode H "Fluoroquinolones: interaction profile during enteral absorption." Drugs 45 Suppl 3 (1993): 65-72. [PMID: 7689454] | ||||
| 22 | Campbell NR, Kara M, Hasinoff BB, Haddara WM, McKay DW "Norfloxacin interaction with antacids and minerals." Br J Clin Pharmacol 33 (1992): 115-6. [PMID: 1540482] | ||||
| 23 | Davis R, Bryson HM "Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy [published erratum appears in Drugs 1994 Jul 48(1):132]." Drugs 47 (1994): 677-700. [PMID: 7516863] | ||||
| 24 | Product Information. Spectracef (cefditoren). TAP Pharmaceuticals Inc, Deerfield, IL. | ||||
| 25 | Product Information. Boniva (ibandronate). Roche Laboratories, Nutley, NJ. | ||||
| 26 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 27 | Bullingham R, Shah J, Goldblum R, Schiff M "Effects of food and antacid on the pharmacokinetics of single doses of mycophenolate mofetil in rheumatoid arthritis patients." Br J Clin Pharmacol 41 (1996): 513-6. [PMID: 8799515] | ||||
| 28 | Chun AH, Carrigan PJ, Hoffman DJ, Kershner RP, Stuart JD "Effect of antacids on absorption of clorazepate." Clin Pharmacol Ther 22 (1977): 329-35. [PMID: 19188] | ||||
| 29 | May DB, Drew RH, Yedinak KC, Bartlett JA "Effect of simultaneous didanosine administration on itraconazole absorption in healthy volunteers." Pharmacotherapy 14 (1994): 509-13. [PMID: 7997384] | ||||
| 30 | Product Information. Rifadin (rifampin). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 31 | Product Information. Accolate (zafirlukast). Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 32 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 33 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 34 | Mantyla R, Mannisto PT, Vuorela A, Sundberg S, Ottoila P "Impairment of captopril bioavailability by concomitant food and antacid intake." Int J Clin Pharmacol Ther Toxicol 22 (1984): 626-9. [PMID: 6389377] | ||||
| 35 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 36 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 37 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 38 | MHRA. Medicines and Healthcare Products Regulatory Agency "Orlistat: theoretical interaction with antiretroviral HIV medicines.". | ||||
| 39 | Mendelson J, Jones RT, Upton R, Jacob P 3rd "Methamphetamine and ethanol interactions in humans." Clin Pharmacol Ther 57 (1995): 559-68. [PMID: 7768079] | ||||
| 40 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 41 | Product Information. Ferriprox (deferiprone). ApoPharma USA Inc, Rockville, MD. | ||||
