Details of the Drug
General Information of Drug (ID: DMIVJ0D)
| Drug Name |
Pseudoephedrine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Balminil Decongestant Syrup; Dimetapp Decongestant; Dimetapp Decongestant Pediatric Drops; Drixoral Nasal Decongestant; Pseudoephedrine Ephedrine; Sudafed Decongestant Extra Strength; Triaminic AM Decongestant Formula; Triaminic Infant Oral Decongestant Drops; Efidac 24 PseudoephedrineHcl; Sudafed Decongestant 12 Hour; Pseudoefedrina [INN-Spanish]; Pseudoephedrine (INN); Pseudoephedrine [INN:BAN]; Pseudoephedrine d-form; Pseudoephedrinum [INN-Latin]; Sudafed (TN); Alpha-(1-(Methylamino)ethyl)benzyl alcohol; D-psi-2-Methylamino-1-phenyl-1-propanol; Benzenemethanol, alpha-((1S)-1-(methylamino)ethyl)-, (alpha-S)-(9CI); (+)-(1S,2S)-Pseudoephedrine; (1S,2S)-(+)-Pseudoephedrine; (1S,2S)-2-(methylamino)-1-phenylpropan-1-ol; (1S,2S)-2-Methylamino-1-phenyl-1-propanol; (1S,2S)-Pseudoephedrine; (1S,2S)-Pseudoephedrine, polymer-bound
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
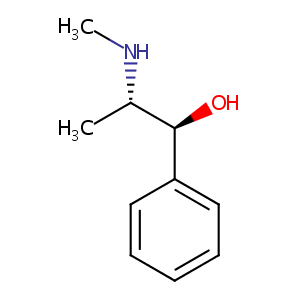 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 165.23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Pseudoephedrine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Ephedrine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7286). | ||||
| 3 | Chao ST, Prather D, Pinson D, Coen P, Pruitt B, Knowles M, Place V: Effect of food on bioavailability of pseudoephedrine and brompheniramine administered from a gastrointestinal therapeutic system. J Pharm Sci. 1991 May;80(5):432-5. doi: 10.1002/jps.2600800507. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | FDA Approved Drug Products: Semprex Avacristine and Pseudoephedrine Oral Capsules | ||||
| 6 | FDA Approved Drug Products: Zyrtec Cetirizine and Pseudoephedrine Oral Tablets (OTC) | ||||
| 7 | Cetirizine/pseudoephedrine. Drugs. 2001;61(15):2231-40; discussion 2241-2. | ||||
| 8 | Benzylic alcohols as stereospecific substrates and inhibitors for aryl sulfotransferase. Chirality. 1991;3(2):104-11. | ||||
| 9 | Brater DC, Kaojarern S, Benet LZ, et al "Renal excretion of pseudoephedrine." Clin Pharmacol Ther 28 (1980): 690-4. [PMID: 7438686] | ||||
| 10 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 11 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 12 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 13 | Product Information. Meridia (sibutramine). Knoll Pharmaceutical Company, Whippany, NJ. | ||||
| 14 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 15 | Reeves RR, Pinkofsky HB "Postpartum psychosis induced by bromocriptine and pseudoephedrine." J Fam Pract 45 (1997): 164-6. [PMID: 9267376] | ||||
| 16 | Barthel W, Glusa E, Koth W "Interactions of dihydroergotamine with etilefrine in human leg veins in vitro and in situ." Int J Clin Pharmacol Ther Toxicol 25 (1987): 63-9. [PMID: 2881898] | ||||
