Details of the Drug
General Information of Drug (ID: DMVE3TK)
| Drug Name |
Bromocriptine
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bagren; Bromergocryptine; Bromocriptin; Bromocriptina; Bromocriptinum; Bromocryptin; Bromocryptine; Bromoergocriptine; Bromoergocryptine; Ergoset; Parlodel; Bromocriptine [BAN]; Bromocriptine methanesulfonate; Parlodel Snaptabs; Alti-Bromocriptine; Apo-Bromocriptine; Bromocriptina [INN-Spanish]; Bromocriptinum [INN-Latin]; CB-154; Parlodel (TN); Bromocriptine (USAN/INN); Bromocriptine [USAN:BAN:INN]; Ergocryptine, 2-bromo-(8CI); (5'alpha)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-3',6',18-trioxoergotaman; (5'alpha)-2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)ergotaman-3',6',18-trione; (5'alpha)-2-bromo-12'-hydroxy-5'-(2-methylpropyl)-2'-(propan-2-yl)-3',6',18-trioxoergotaman; (5'alpha)-2-bromo-12'-hydroxy-5'-isobutyl-2'-isopropyl-3',6',18-trioxoergotaman; (6aR,9R)-5-Bromo-N-((2R,5S,10aS,10bS)-10b-hydroxy-5-isobutyl-2-isopropyl-3,6-dioxooctahydro-2H-oxazolo[3,2-a]pyrrolo[2,1-c]pyrazin-2-yl)-7-methyl-4,6,6a,7,8,9-hexahydroindolo[4,3-fg]quinoline-9-carboxamide; 2-Bromo-12'-hydroxy-2'-(1-methylethyl)-5'-alpha-(2-methylpropyl)ergotamin-3',6',18-trione; 2-Bromo-alpha-ergocryptine; 2-Bromo-alpha-ergokryptin; 2-Bromo-alpha-ergokryptine; 2-Bromoergocryptine Methanesulfonate; 2-Bromoergokryptine
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiparkinson Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
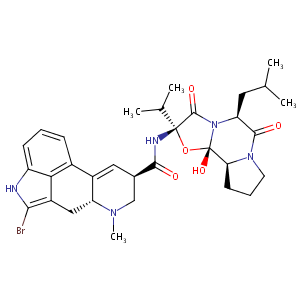 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 654.6 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acromegaly | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A60.0 | |||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Bromocriptine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Bromocriptine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 35). | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Silymarin BIO-C, an extract from Silybum marianum fruits, induces hyperprolactinemia in intact female rats. Phytomedicine. 2009 Sep;16(9):839-44. | ||||
| 7 | Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109. | ||||
| 8 | Kinetics of dithionite-dependent reduction of cytochrome P450 3A4: heterogeneity of the enzyme caused by its oligomerization. Biochemistry. 2005 Oct 25;44(42):13902-13. | ||||
| 9 | Bromocriptine methylate suppresses glial inflammation and moderates disease progression in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2011 Nov;232(1):41-52. doi: 10.1016/j.expneurol.2011.08.001. Epub 2011 Aug 16. | ||||
| 10 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 11 | Mechanism of interactions of alpha-naphthoflavone with cytochrome P450 3A4 explored with an engineered enzyme bearing a fluorescent probe. Biochemistry. 2007 Jan 9;46(1):106-19. doi: 10.1021/bi061944p. | ||||
| 12 | Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neurosci Lett. 2006 Sep 25;405(3):196-201. doi: 10.1016/j.neulet.2006.07.030. Epub 2006 Aug 8. | ||||
| 13 | Plasma dopamine beta hydroxylase (D.B.H.) activity in Parkinsonian patients under L-dopa, and 2-bromo-alpha-ergocriptine loading. J Neural Transm. 1979;46(1):71-8. doi: 10.1007/BF01243430. | ||||
| 14 | Resolution of hyperprolactinaemia after bromocriptine-induced pregnancy. Lancet. 1979 Apr 7;1(8119):784-5. doi: 10.1016/s0140-6736(79)91247-9. | ||||
| 15 | Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol Endocrinol Metab. 2006 Nov;291(5):E1038-43. doi: 10.1152/ajpendo.00567.2005. Epub 2006 Jun 27. | ||||
| 16 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 19 | Flogstad AK, Halse J, Grass P, Abisch E, Djoseland O, Kutz K, Bodd E, Jervell J, Flgstad AK, Djseland O "A comparison of octreotide, bromocriptine, or a combination of both drugs in acromegaly." J Clin Endocrinol Metab 79 (1994): 461-5. [PMID: 8045964] | ||||
| 20 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 21 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 22 | Mims RB, Scott CL, Modebe O, Bethune JE "Inhibition of L-dopa-induced growth hormone stimulation by pyridoxine and chlorpromazine." J Clin Endocrinol Metab 40 (1975): 256-9. [PMID: 1117978] | ||||
| 23 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 24 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 25 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 26 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 27 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 28 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 29 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 30 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 31 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 32 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 33 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 34 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 35 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 36 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 37 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 38 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 39 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 40 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 41 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 42 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 43 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 44 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 45 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 46 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 47 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 48 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 49 | Product Information. Norprolac (quinagolide). Ferring Inc, North York, IA. | ||||
