Details of the Drug
General Information of Drug (ID: DMMV0KW)
| Drug Name |
Ephedrine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Biophedrin; Eciphin; Efedrin; Ephedral; Ephedremal; Ephedrin; Ephedrital; Ephedrol; Ephedrosan; Ephedrotal; Ephedsol; Ephendronal; Ephoxamin; Fedrin; Kratedyn; Lexofedrin; Manadrin; Mandrin; Nasol; Racephedrine; Sanedrine; Vencipon; Zephrol; CPDD 0049; D-Ephedrine; Ephedrine (TN); Ephedrine (USP); Ephedrine [USAN:BAN]; Ephedrine l-form; I-Sedrin; L-Ephedrine; L(-)-Ephedrine; L-(+)-Ephedrine; L-(-)-Ephedrine; (+)-Ephedrin; (1R,2R)-Ephedrine; (1S,2R)-Ephedrine; (L)-EPHEDRINE; 1-EPHEDRINE; 1-Sedrin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Central Nervous System Stimulants
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
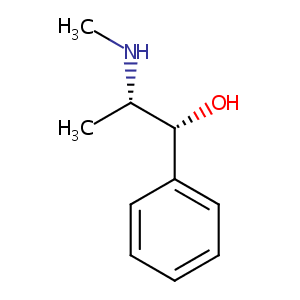 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 165.23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Allergic rhinitis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA08.0 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ephedrine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Ephedrine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 556). | ||||
| 3 | The pharmacokinetics of ephedrine after oral dosage in asthmatics receiving acute and chronic treatment. Br J Clin Pharmacol. 1976 Feb;3(1):123-34. doi: 10.1111/j.1365-2125.1976.tb00579.x. | ||||
| 4 | Sever PS, Dring LG, Williams RT: The metabolism of (-)-ephedrine in man. Eur J Clin Pharmacol. 1975 Dec 19;9(2-3):193-8. doi: 10.1007/bf00614017. | ||||
| 5 | The effect of alpha-2 adrenergic agonists on memory and cognitive flexibility. Cogn Behav Neurol. 2006 Dec;19(4):204-7. | ||||
| 6 | Benzylic alcohols as stereospecific substrates and inhibitors for aryl sulfotransferase. Chirality. 1991;3(2):104-11. | ||||
| 7 | Selection of drugs to test the specificity of the Tg.AC assay by screening for induction of the gadd153 promoter in vitro. Toxicol Sci. 2003 Aug;74(2):260-70. doi: 10.1093/toxsci/kfg113. Epub 2003 May 2. | ||||
| 8 | Brater DC, Kaojarern S, Benet LZ, et al "Renal excretion of pseudoephedrine." Clin Pharmacol Ther 28 (1980): 690-4. [PMID: 7438686] | ||||
| 9 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 10 | Product Information. Northera (droxidopa). Chelsea Therapeutics Inc, Charlotte, NC. | ||||
| 11 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 12 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 13 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 14 | Achor MB, Extein I "Diet aids, mania, and affective illness" Am J Psychiatry 138 (1981): 392. [PMID: 7468847] | ||||
