Details of the Drug
General Information of Drug (ID: DMUSZHD)
| Drug Name |
Hydrochlorothiazide
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acesistem; Acuilix; Acuretic; Aldazida; Aldoril; Apresazide; Aquarills; Aquarius; Bremil; Briazide; Caplaril; Carozide; Catiazida; Chlorizide; Chlorosulthiadil; Chlorzide; Chlothia; Cidrex; Clorana; Condiuren; Diaqua; Dichlorosal; Dichlorotride; Dichlothiazide; Dichlotiazid; Dichlotride; Diclotride; Dicyclotride; Didral; Dihydran; Dihydrochlorothiazid; Dihydrochlorothiazide; Dihydrochlorothiazidum; Dihydrochlorurit; Dihydrochlorurite; Dihydroxychlorothiazidum; Direma; Disalunil; Disothiazid; Diurogen; Dixidrasi; Drenol; Esidrex; Esidrix; Esoidrina; Fluvin; HCT; HCTZ; HCZ; Hidril; Hidrochlortiazid; Hidroclorotiazida; Hidroronol; Hidrosaluretil; Hidrotiazida; Hyclosid; Hydril; HydroDIURIL; Hydrochlorat; Hydrochlorot; Hydrochlorothiazid; Hydrochlorothiazidum; Hydrochlorthiazide; Hydrochlorthiazidum; Hydrocot; Hydrodiuretic; Hydropres; Hydrosaluric; Hydrothide; Hydrozide; Hypothiazid; Hypothiazide; Hytrid; Idroclorotiazide; Idrotiazide; Indroclor; Ivaugan; Manuril; Maschitt; Medozide; Megadiuril; Microzide; Mictrin; Mikorten; Modurcen; Moduretic; Natrinax; Nefrix; Nefrol; Neoflumen; Newtolide; Novodiurex; Oretic; Pantemon; Panurin; Roxane; Saldiuril; Sectrazide; Selozide; Servithiazid; Spironazide; Tandiur; Thiaretic; Thiuretic; Thlaretic; Timolide; Unazid; Urodiazin; Urozide; Vaseretic; Vetidrex; Aquazide H; Chlorsulfonamidodihydrobenzothiadiazine dioxide; Component of Aldactazide; Component of Aldoril; Component of Butizide Prestabs; Component of Caplaril; Component of Cyclex; Component of Dyazide; Component of Esimil; Concor Plus; Diclot ride; Hydro Par; Hydrochlorothiazide Intensol; Idroclorotiazide [DCIT]; Lotensin HCT; Panurin dichloride; Raunova Plus; Diu 25 Vigt; H 4759; MaybridgeCompound 10; Mazide 25 mg; Su 5879; Aldactazide 25/25; Aldectazide 50/50; Apo-Hydro; Aquazide H (TN); Aquazide-H; Dichlotride (TN); Diu-melusin; Esidrex (TN); Esidrix (TN); HCT-Isis; Hidro-Niagrin; Hidroclorotiazida [INN-Spanish]; Hydrex-semi; Hydro-Aquil; Hydro-D; Hydro-Diuril; Hydro-Saluric; Hydro-T; Hydro-chlor; HydroSaluric (TN); Hydrochlorothiazidum [INN-Latin]; Hydrodiuril (TN); Hydrozide Injection, Veterinary; Jen-Diril; Microzide (TN); Neo-Flumen; Neo-Minzil; Neo-codema; Oretic (TN); Ro-hydrazide; AF-614/30832002; Apo-Hydro (TN); Hydrochlorothiazide [INN:BAN:JAN]; Hydrochlorothiazide (JP15/USP/INN); Dro-2H-1,2,4-benzothiadiazine 1,1-dioxide; 3,4-Dihydro-6-chloro-7-sulfamyl-1,2, 4-benzothi; 3,4-Dihydrochlorothiazide; 6-Chloro-7-sulfamoyl-3, 4-dihy
|
|||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Diuretics
|
|||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||
| Structure |
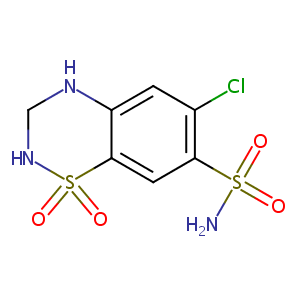 |
|||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 297.7 | ||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.1 | |||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | |||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic heart failure | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BD1Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Hydrochlorothiazide
Coadministration of a Drug Treating the Disease Different from Hydrochlorothiazide (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Hydrochlorothiazide FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4836). | ||||
| 3 | FDA Approved Drug Products: Hydrochlorothiazide Oral Capsules | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Niemeyer C, Hasenfuss G, Wais U, Knauf H, Schafer-Korting M, Mutschler E: Pharmacokinetics of hydrochlorothiazide in relation to renal function. Eur J Clin Pharmacol. 1983;24(5):661-5. doi: 10.1007/bf00542218. | ||||
| 6 | FDA Approved Drug Products: Hydrochlorothiazide Oral Tablets | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Chen TM, Chiou WL: Large differences in the biological half-life and volume of distribution of hydrochlorothiazide in normal subjects from eleven studies. Correlation with their last blood sampling times. Int J Clin Pharmacol Ther Toxicol. 1992 Jan;30(1):34-7. | ||||
| 9 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 10 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 11 | Multichannel liquid chromatography-tandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007 Dec;24(12):2281-96. | ||||
| 12 | Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3. | ||||
| 13 | Dean S, Kendall MJ, Potter S, Thompson MH, Jackson DA "Nadolol in combination with indapamide and xipamide in resistant hypertensives." Eur J Clin Pharmacol 28 (1985): 29-33. [PMID: 3987783] | ||||
| 14 | Burnakis TG, Mioduch HJ "Combined therapy with captopril and potassium supplementation: a potential for hyperkalemia." Arch Intern Med 144 (1984): 2371-2. [PMID: 6391404] | ||||
| 15 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 16 | FDA. U.S. Food and Drug Administration "FDA Drug Safety Communication: Low magnesium levels can be associated with long-term use of proton pump inhibitor drugs (PPIs).". | ||||
| 17 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 18 | McCarthy JT, Torres VE, Romero JC, et al "Acute intrinsic renal failure induced by indomethacin." Mayo Clin Proc 57 (1982): 289-96. [PMID: 6952058] | ||||
| 19 | Product Information. Savella (milnacipran). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 20 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 21 | Beermann B, Groschinsky-Grind M "Enhancement of the gastrointestinal absorption of hydrochlorothiazide by propantheline." Eur J Clin Pharmacol 13 (1978): 385-7. [PMID: 668798] | ||||
| 22 | Product Information. Aptiom (eslicarbazepine). Sunovion Pharmaceuticals Inc, Marlborough, MA. | ||||
| 23 | Brown DD, Dormois JC, Abraham GN, et al "Effect of furosemide on the renal excretion of digoxin." Clin Pharmacol Ther 20 (1976): 395-400. [PMID: 975715] | ||||
| 24 | Ohnishi K, Yoshida H, Shigeno K, et al. "Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia." Ann Intern Med 133 (2000): 881-5. [PMID: 11103058] | ||||
| 25 | Cohen J "Long-term efficacy and safety of terazosin alone and in combination with other antihypertensive agents." Am Heart J 122 (1991): 919-25. [PMID: 1678923] | ||||
| 26 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 27 | Product Information. Orap Tablets (pimozide). Gate Pharmaceuticals, Sellersville, PA. | ||||
| 28 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 29 | Antonelli D, Atar S, Freedberg NA, Rosenfeld T "Torsade de pointes in patients on chronic amiodarone treatment: contributing factors and drug interactions." Isr Med Assoc J 7 (2005): 163-5. [PMID: 15792261] | ||||
