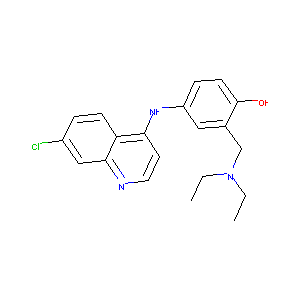
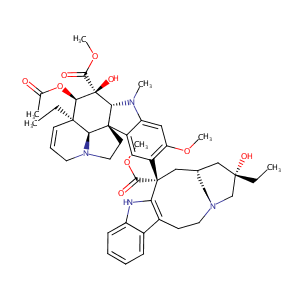
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Multidrug resistance-associated protein 1 (ABCC1)
|
OTGUN89S
|
MRP1_HUMAN
|
Decreases Response To Substance
|
[28] |
|
ATP-dependent translocase ABCB1 (ABCB1)
|
OTEJROBO
|
MDR1_HUMAN
|
Decreases Response To Substance
|
[29] |
|
ATP-binding cassette sub-family C member 2 (ABCC2)
|
OTJSIGV5
|
MRP2_HUMAN
|
Affects Transport
|
[30] |
|
Bile salt export pump (ABCB11)
|
OTRU7THO
|
ABCBB_HUMAN
|
Decreases Activity
|
[31] |
|
Inhibitor of nuclear factor kappa-B kinase subunit beta (IKBKB)
|
OT9RDS3H
|
IKKB_HUMAN
|
Increases Phosphorylation
|
[32] |
|
Nuclear receptor subfamily 1 group I member 2 (NR1I2)
|
OTC5U0N5
|
NR1I2_HUMAN
|
Increases Activity
|
[33] |
|
Superoxide dismutase , mitochondrial (SOD2)
|
OTIWXGZ9
|
SODM_HUMAN
|
Increases Expression
|
[34] |
|
Interleukin-6 (IL6)
|
OTUOSCCU
|
IL6_HUMAN
|
Increases Stability
|
[35] |
|
Intercellular adhesion molecule 1 (ICAM1)
|
OTTOIX77
|
ICAM1_HUMAN
|
Decreases Expression
|
[36] |
|
Transcription factor Jun (JUN)
|
OTCYBO6X
|
JUN_HUMAN
|
Increases Phosphorylation
|
[37] |
|
Interleukin-8 (CXCL8)
|
OTS7T5VH
|
IL8_HUMAN
|
Increases Stability
|
[35] |
|
Integrin alpha-L (ITGAL)
|
OTCUQAIS
|
ITAL_HUMAN
|
Decreases Expression
|
[36] |
|
NF-kappa-B inhibitor alpha (NFKBIA)
|
OTFT924M
|
IKBA_HUMAN
|
Increases Phosphorylation
|
[32] |
|
Mitogen-activated protein kinase 8 (MAPK8)
|
OTEREYS5
|
MK08_HUMAN
|
Increases Phosphorylation
|
[37] |
|
Mitogen-activated protein kinase 9 (MAPK9)
|
OTCEVJ9E
|
MK09_HUMAN
|
Increases Phosphorylation
|
[37] |
|
Tumor necrosis factor ligand superfamily member 6 (FASLG)
|
OTZARCHH
|
TNFL6_HUMAN
|
Increases Expression
|
[38] |
|
Protein kinase C delta type (PRKCD)
|
OTSEH90E
|
KPCD_HUMAN
|
Affects Localization
|
[34] |
|
Nuclear factor of activated T-cells, cytoplasmic 4 (NFATC4)
|
OTTDCUAO
|
NFAC4_HUMAN
|
Decreases Localization
|
[39] |
|
Transcriptional regulator QRICH1 (QRICH1)
|
OTPVAX04
|
QRIC1_HUMAN
|
Increases Phosphorylation
|
[40] |
|
Sorting nexin-10 (SNX10)
|
OT05B7BT
|
SNX10_HUMAN
|
Affects Response To Substance
|
[18] |
|
Histone H1.2 (H1-2)
|
OT0AVI4M
|
H12_HUMAN
|
Affects Response To Substance
|
[18] |
|
Interleukin-1 beta (IL1B)
|
OT0DWXXB
|
IL1B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Friend leukemia integration 1 transcription factor (FLI1)
|
OT0EV3LX
|
FLI1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Sulfotransferase 1A1 (SULT1A1)
|
OT0K7JIE
|
ST1A1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Histone H3-like centromeric protein A (CENPA)
|
OT0NEJ4X
|
CENPA_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA polymerase zeta catalytic subunit (REV3L)
|
OT0OP8EJ
|
REV3L_HUMAN
|
Affects Response To Substance
|
[18] |
|
Rab11 family-interacting protein 1 (RAB11FIP1)
|
OT0T71OW
|
RFIP1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Oxysterol-binding protein-related protein 10 (OSBPL10)
|
OT0TFMBE
|
OSB10_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA-binding protein inhibitor ID-2 (ID2)
|
OT0U1D53
|
ID2_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase 1 alpha subcomplex subunit 13 (NDUFA13)
|
OT0UOKIT
|
NDUAD_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tripartite motif-containing protein 2 (TRIM2)
|
OT0V1YVC
|
TRIM2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Lamin-B1 (LMNB1)
|
OT100T3P
|
LMNB1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Borealin (CDCA8)
|
OT17D55D
|
BOREA_HUMAN
|
Affects Response To Substance
|
[18] |
|
G2/mitotic-specific cyclin-B1 (CCNB1)
|
OT19S7E5
|
CCNB1_HUMAN
|
Increases Response To Substance
|
[41] |
|
Condensin complex subunit 3 (NCAPG)
|
OT1AI9EO
|
CND3_HUMAN
|
Affects Response To Substance
|
[18] |
|
m7GpppX diphosphatase (DCPS)
|
OT1FZVC9
|
DCPS_HUMAN
|
Affects Response To Substance
|
[18] |
|
KN motif and ankyrin repeat domain-containing protein 1 (KANK1)
|
OT2E7A6W
|
KANK1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Aldehyde oxidase (AOX1)
|
OT2FZP6H
|
AOXA_HUMAN
|
Affects Response To Substance
|
[18] |
|
U11/U12 small nuclear ribonucleoprotein 25 kDa protein (SNRNP25)
|
OT2MR6O2
|
SNR25_HUMAN
|
Affects Response To Substance
|
[18] |
|
Pyruvate dehydrogenase E1 component subunit beta, mitochondrial (PDHB)
|
OT2NHE5E
|
ODPB_HUMAN
|
Affects Response To Substance
|
[18] |
|
N-acetylglucosamine-1-phosphotransferase subunits alpha/beta (GNPTAB)
|
OT2Z03OB
|
GNPTA_HUMAN
|
Affects Response To Substance
|
[18] |
|
Superoxide dismutase (SOD1)
|
OT39TA1L
|
SODC_HUMAN
|
Affects Response To Substance
|
[18] |
|
Baculoviral IAP repeat-containing protein 3 (BIRC3)
|
OT3E95KB
|
BIRC3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Chromosome-associated kinesin KIF4A (KIF4A)
|
OT3UWL7D
|
KIF4A_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase 1 beta subcomplex subunit 2, mitochondrial (NDUFB2)
|
OT4717TF
|
NDUB2_HUMAN
|
Affects Response To Substance
|
[18] |
|
High mobility group protein B1 (HMGB1)
|
OT4B7CPF
|
HMGB1_HUMAN
|
Affects Response To Substance
|
[18] |
|
c-Myc-binding protein (MYCBP)
|
OT4IZR4R
|
MYCBP_HUMAN
|
Affects Response To Substance
|
[18] |
|
Hyaluronan mediated motility receptor (HMMR)
|
OT4M0JTZ
|
HMMR_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transcription termination factor 2 (TTF2)
|
OT5LJOWM
|
TTF2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Neprilysin (MME)
|
OT5Q39P8
|
NEP_HUMAN
|
Affects Response To Substance
|
[18] |
|
Peptidyl-prolyl cis-trans isomerase FKBP1A (FKBP1A)
|
OT6285L4
|
FKB1A_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA topoisomerase 2-alpha (TOP2A)
|
OT6LPS08
|
TOP2A_HUMAN
|
Affects Response To Substance
|
[18] |
|
Electron transfer flavoprotein subunit beta (ETFB)
|
OT6Q6FBD
|
ETFB_HUMAN
|
Affects Response To Substance
|
[18] |
|
Flap endonuclease 1 (FEN1)
|
OT6QGG7O
|
FEN1_HUMAN
|
Affects Response To Substance
|
[18] |
|
G-protein-signaling modulator 2 (GPSM2)
|
OT6RPMRM
|
GPSM2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Plasminogen activator inhibitor 2 (SERPINB2)
|
OT72QLZB
|
PAI2_HUMAN
|
Affects Response To Substance
|
[18] |
|
CD40 ligand (CD40LG)
|
OT75Z6A6
|
CD40L_HUMAN
|
Decreases Response To Substance
|
[42] |
|
Mitotic checkpoint serine/threonine-protein kinase BUB1 (BUB1)
|
OT80DZMT
|
BUB1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Nucleolar and spindle-associated protein 1 (NUSAP1)
|
OT85HIJ5
|
NUSAP_HUMAN
|
Affects Response To Substance
|
[18] |
|
Stanniocalcin-2 (STC2)
|
OT86YP8G
|
STC2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Pirin (PIR)
|
OT8ALXHU
|
PIR_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase 1 alpha subcomplex subunit 7 (NDUFA7)
|
OT8CV3H5
|
NDUA7_HUMAN
|
Affects Response To Substance
|
[18] |
|
G0/G1 switch protein 2 (G0S2)
|
OT8FL49L
|
G0S2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Replication protein A 14 kDa subunit (RPA3)
|
OT8JAQGL
|
RFA3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Deoxyribose-phosphate aldolase (DERA)
|
OT8SMO3N
|
DEOC_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase iron-sulfur protein 6, mitochondrial (NDUFS6)
|
OT9IOONQ
|
NDUS6_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase 1 subunit C2 (NDUFC2)
|
OT9M119L
|
NDUC2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Beta-glucuronidase (GUSB)
|
OT9N1DK3
|
BGLR_HUMAN
|
Affects Response To Substance
|
[18] |
|
Fos-related antigen 1 (FOSL1)
|
OT9YTYMB
|
FOSL1_HUMAN
|
Affects Response To Substance
|
[18] |
|
High mobility group nucleosome-binding domain-containing protein 3 (HMGN3)
|
OTA2TBWS
|
HMGN3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Diphosphoinositol polyphosphate phosphohydrolase NUDT4B (NUDT4)
|
OTA6ICI2
|
NUD4B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cysteine-rich PDZ-binding protein (CRIPT)
|
OTA6M8M4
|
CRIPT_HUMAN
|
Affects Response To Substance
|
[18] |
|
Replication factor C subunit 5 (RFC5)
|
OTAD79RT
|
RFC5_HUMAN
|
Affects Response To Substance
|
[18] |
|
ATP synthase subunit e, mitochondrial (ATP5ME)
|
OTADVEE2
|
ATP5I_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transmembrane protein 14A (TMEM14A)
|
OTANQA6G
|
TM14A_HUMAN
|
Affects Response To Substance
|
[18] |
|
MAP kinase-activated protein kinase 5 (MAPKAPK5)
|
OTAZHPVK
|
MAPK5_HUMAN
|
Affects Response To Substance
|
[18] |
|
Shootin-1 (SHTN1)
|
OTB07MOG
|
SHOT1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cyclin-dependent kinase 2 (CDK2)
|
OTB5DYYZ
|
CDK2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Fibronectin (FN1)
|
OTB5ZN4Q
|
FINC_HUMAN
|
Affects Response To Substance
|
[18] |
|
Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3)
|
OTB97VIK
|
IF2B3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Membrane-associated progesterone receptor component 1 (PGRMC1)
|
OTBE6WAC
|
PGRC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Probable ATP-dependent RNA helicase DDX23 (DDX23)
|
OTBJHS8C
|
DDX23_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein FAM3C (FAM3C)
|
OTBR6U9G
|
FAM3C_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transmembrane protein 134 (TMEM134)
|
OTBX9H9D
|
TM134_HUMAN
|
Affects Response To Substance
|
[18] |
|
Dual specificity protein kinase TTK (TTK)
|
OTBY1Z5H
|
TTK_HUMAN
|
Affects Response To Substance
|
[18] |
|
CCN family member 2 (CCN2)
|
OTC39NSU
|
CCN2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cyclin-dependent kinase 4 inhibitor C (CDKN2C)
|
OTCLOV90
|
CDN2C_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cytoskeleton-associated protein 2 (CKAP2)
|
OTCLTC0J
|
CKAP2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cytochrome c oxidase copper chaperone (COX17)
|
OTCP4LH2
|
COX17_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tetraspanin-13 (TSPAN13)
|
OTCS9BZY
|
TSN13_HUMAN
|
Affects Response To Substance
|
[18] |
|
S-phase kinase-associated protein 2 (SKP2)
|
OTD5B41X
|
SKP2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Succinate--CoA ligase subunit alpha, mitochondrial (SUCLG1)
|
OTDCSPXH
|
SUCA_HUMAN
|
Affects Response To Substance
|
[18] |
|
Proteasome activator complex subunit 4 (PSME4)
|
OTDGF53O
|
PSME4_HUMAN
|
Affects Response To Substance
|
[18] |
|
Gamma-adducin (ADD3)
|
OTDRSHAZ
|
ADDG_HUMAN
|
Affects Response To Substance
|
[18] |
|
Superkiller complex protein 8 (SKIC8)
|
OTDSQYDN
|
SKI8_HUMAN
|
Affects Response To Substance
|
[18] |
|
Dickkopf-related protein 3 (DKK3)
|
OTDU94EY
|
DKK3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Geminin (GMNN)
|
OTDYKNIY
|
GEMI_HUMAN
|
Affects Response To Substance
|
[18] |
|
EF-hand calcium-binding domain-containing protein 7 (EFCAB7)
|
OTE0EG10
|
EFCB7_HUMAN
|
Affects Response To Substance
|
[18] |
|
Vitamin K-dependent gamma-carboxylase (GGCX)
|
OTE0FNAP
|
VKGC_HUMAN
|
Affects Response To Substance
|
[18] |
|
ATP synthase subunit f, mitochondrial (ATP5MF)
|
OTE4TBG9
|
ATPK_HUMAN
|
Affects Response To Substance
|
[18] |
|
Maternal embryonic leucine zipper kinase (MELK)
|
OTEY2UQA
|
MELK_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein pelota homolog (PELO)
|
OTF3WLIM
|
PELO_HUMAN
|
Affects Response To Substance
|
[18] |
|
Serglycin (SRGN)
|
OTFAVSX0
|
SRGN_HUMAN
|
Affects Response To Substance
|
[18] |
|
Sortilin (SORT1)
|
OTFCQ4B1
|
SORT_HUMAN
|
Affects Response To Substance
|
[18] |
|
Exosome complex component RRP45 (EXOSC9)
|
OTFKB37F
|
EXOS9_HUMAN
|
Affects Response To Substance
|
[18] |
|
Baculoviral IAP repeat-containing protein 2 (BIRC2)
|
OTFXFREP
|
BIRC2_HUMAN
|
Affects Response To Substance
|
[18] |
|
High mobility group protein B2 (HMGB2)
|
OTGEGAOK
|
HMGB2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Phospholipid-transporting ATPase ABCA3 (ABCA3)
|
OTH6MSKQ
|
ABCA3_HUMAN
|
Decreases Response To Substance
|
[43] |
|
Sigma intracellular receptor 2 (TMEM97)
|
OTH76ZWK
|
SGMR2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transmembrane protein 156 (TMEM156)
|
OTH7YJID
|
TM156_HUMAN
|
Affects Response To Substance
|
[18] |
|
Epithelial cell adhesion molecule (EPCAM)
|
OTHBZK5X
|
EPCAM_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein regulator of cytokinesis 1 (PRC1)
|
OTHD0XS0
|
PRC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Catalase (CAT)
|
OTHEBX9R
|
CATA_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kinesin-like protein KIF11 (KIF11)
|
OTHRGLCQ
|
KIF11_HUMAN
|
Affects Response To Substance
|
[18] |
|
Actin-binding LIM protein 1 (ABLIM1)
|
OTHXEK3E
|
ABLM1_HUMAN
|
Affects Response To Substance
|
[18] |
|
U6 snRNA-associated Sm-like protein LSm7 (LSM7)
|
OTHZ2XPX
|
LSM7_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kinesin-like protein KIF1B (KIF1B)
|
OTI1XQTO
|
KIF1B_HUMAN
|
Affects Response To Substance
|
[18] |
|
Interstitial collagenase (MMP1)
|
OTI4I2V1
|
MMP1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein Mis18-beta (OIP5)
|
OTI5C2DE
|
MS18B_HUMAN
|
Affects Response To Substance
|
[18] |
|
G2/mitotic-specific cyclin-B2 (CCNB2)
|
OTIEXTDK
|
CCNB2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Securin (PTTG1)
|
OTIMYS4W
|
PTTG1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Baculoviral IAP repeat-containing protein 7 (BIRC7)
|
OTITCGHB
|
BIRC7_HUMAN
|
Decreases Response To Substance
|
[44] |
|
Aurora kinase B (AURKB)
|
OTIY4VHU
|
AURKB_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase 1 subunit C1, mitochondrial (NDUFC1)
|
OTJ5TNHB
|
NDUC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Low molecular weight phosphotyrosine protein phosphatase (ACP1)
|
OTJ9CKLU
|
PPAC_HUMAN
|
Affects Response To Substance
|
[18] |
|
G/T mismatch-specific thymine DNA glycosylase (TDG)
|
OTJB0YIH
|
TDG_HUMAN
|
Affects Response To Substance
|
[18] |
|
E3 ubiquitin-protein ligase FANCL (FANCL)
|
OTJC7QPQ
|
FANCL_HUMAN
|
Affects Response To Substance
|
[18] |
|
Hydroxyacyl-coenzyme A dehydrogenase, mitochondrial (HADH)
|
OTJDOL20
|
HCDH_HUMAN
|
Affects Response To Substance
|
[18] |
|
Lysophosphatidic acid receptor 1 (LPAR1)
|
OTJNJQPF
|
LPAR1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kinesin-like protein KIF15 (KIF15)
|
OTJRJEXL
|
KIF15_HUMAN
|
Affects Response To Substance
|
[18] |
|
TBC1 domain family member 4 (TBC1D4)
|
OTK66C40
|
TBCD4_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tyrosine-protein kinase receptor UFO (AXL)
|
OTKA2SUX
|
UFO_HUMAN
|
Affects Response To Substance
|
[18] |
|
Gamma-glutamylcyclotransferase (GGCT)
|
OTKAICP5
|
GGCT_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase 1 alpha subcomplex subunit 1 (NDUFA1)
|
OTKBUQXP
|
NDUA1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Origin recognition complex subunit 6 (ORC6)
|
OTKQN3KP
|
ORC6_HUMAN
|
Affects Response To Substance
|
[18] |
|
Sideroflexin-1 (SFXN1)
|
OTL66767
|
SFXN1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Lamina-associated polypeptide 2, isoform alpha (TMPO)
|
OTL68EL4
|
LAP2A_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transcription factor E2F1 (E2F1)
|
OTLKYBBC
|
E2F1_HUMAN
|
Increases Response To Substance
|
[41] |
|
Striatin (STRN)
|
OTLOZL5I
|
STRN_HUMAN
|
Affects Response To Substance
|
[18] |
|
Succinate--CoA ligase subunit beta, mitochondrial (SUCLA2)
|
OTMZD4PW
|
SUCB1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein downstream neighbor of Son (DONSON)
|
OTN5HE0W
|
DONS_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cytochrome c oxidase subunit 6B1 (COX6B1)
|
OTNKXYQI
|
CX6B1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Keratinocyte-associated transmembrane protein 2 (C5ORF15)
|
OTO126A1
|
KCT2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Receptor tyrosine-protein kinase erbB-2 (ERBB2)
|
OTOAUNCK
|
ERBB2_HUMAN
|
Affects Response To Substance
|
[45] |
|
tRNA (TRMT5)
|
OTOAZBPD
|
TRM5_HUMAN
|
Affects Response To Substance
|
[18] |
|
Ribonucleoside-diphosphate reductase subunit M2 (RRM2)
|
OTOB6J6R
|
RIR2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Cytochrome c oxidase subunit 5A, mitochondrial (COX5A)
|
OTP0961M
|
COX5A_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA fragmentation factor subunit beta (DFFB)
|
OTPBW5N5
|
DFFB_HUMAN
|
Affects Response To Substance
|
[18] |
|
Interleukin-13 receptor subunit alpha-2 (IL13RA2)
|
OTPC2G0X
|
I13R2_HUMAN
|
Affects Response To Substance
|
[18] |
|
DNA dC->dU-editing enzyme APOBEC-3C (APOBEC3C)
|
OTPL0AI1
|
ABC3C_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kazrin (KAZN)
|
OTPM7BYM
|
KAZRN_HUMAN
|
Affects Response To Substance
|
[18] |
|
G2 and S phase-expressed protein 1 (GTSE1)
|
OTPP742Z
|
GTSE1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Prostaglandin E synthase 3 (PTGES3)
|
OTPPQWI0
|
TEBP_HUMAN
|
Affects Response To Substance
|
[18] |
|
Epithelial membrane protein 2 (EMP2)
|
OTPS2H0L
|
EMP2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Ornithine decarboxylase antizyme 1 (OAZ1)
|
OTPT0PKZ
|
OAZ1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Sister chromatid cohesion protein DCC1 (DSCC1)
|
OTPUVLZT
|
DCC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Myc proto-oncogene protein (MYC)
|
OTPV5LUK
|
MYC_HUMAN
|
Affects Response To Substance
|
[46] |
|
Pentraxin-related protein PTX3 (PTX3)
|
OTPXHRKU
|
PTX3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Ran-specific GTPase-activating protein (RANBP1)
|
OTQE226K
|
RANG_HUMAN
|
Affects Response To Substance
|
[18] |
|
Rac GTPase-activating protein 1 (RACGAP1)
|
OTQE8IEH
|
RGAP1_HUMAN
|
Affects Response To Substance
|
[18] |
|
MAPK/MAK/MRK overlapping kinase (MOK)
|
OTQK7M9V
|
MOK_HUMAN
|
Affects Response To Substance
|
[18] |
|
3-oxo-5-alpha-steroid 4-dehydrogenase 1 (SRD5A1)
|
OTQRET2B
|
S5A1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Solute carrier family 22 member 3 (SLC22A3)
|
OTQYGVXX
|
S22A3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Large ribosomal subunit protein mL63 (MRPL57)
|
OTRB659E
|
RT63_HUMAN
|
Affects Response To Substance
|
[18] |
|
Dickkopf-related protein 1 (DKK1)
|
OTRDLUSP
|
DKK1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Olfactory receptor 1I1 (OR1I1)
|
OTRFD8BH
|
OR1I1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein CREG1 (CREG1)
|
OTRHJ8HK
|
CREG1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Proepiregulin (EREG)
|
OTRM4NQY
|
EREG_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kinetochore protein NDC80 homolog (NDC80)
|
OTS7D306
|
NDC80_HUMAN
|
Affects Response To Substance
|
[18] |
|
Latent-transforming growth factor beta-binding protein 2 (LTBP2)
|
OTS88GSD
|
LTBP2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tumor protein D53 (TPD52L1)
|
OTSA6U0I
|
TPD53_HUMAN
|
Affects Response To Substance
|
[18] |
|
Inhibin beta A chain (INHBA)
|
OTSP64PQ
|
INHBA_HUMAN
|
Affects Response To Substance
|
[18] |
|
Leucine-rich repeat-containing protein 1 (LRRC1)
|
OTSVD30Q
|
LRRC1_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase flavoprotein 2, mitochondrial (NDUFV2)
|
OTSZF7D6
|
NDUV2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Plasminogen activator inhibitor 1 (SERPINE1)
|
OTT0MPQ3
|
PAI1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Rho GTPase-activating protein SYDE1 (SYDE1)
|
OTTIAF4D
|
SYDE1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Isocitrate dehydrogenase , mitochondrial (IDH2)
|
OTTQA4PB
|
IDHP_HUMAN
|
Affects Response To Substance
|
[18] |
|
Importin subunit alpha-1 (KPNA2)
|
OTU7FOE6
|
IMA1_HUMAN
|
Affects Response To Substance
|
[18] |
|
General transcription factor II-I (GTF2I)
|
OTUYL1TQ
|
GTF2I_HUMAN
|
Affects Response To Substance
|
[18] |
|
Integrin beta-1-binding protein 1 (ITGB1BP1)
|
OTVQFNGS
|
ITBP1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Transcription factor IIIA (GTF3A)
|
OTVROUVQ
|
TF3A_HUMAN
|
Affects Response To Substance
|
[18] |
|
ATP-binding cassette sub-family A member 2 (ABCA2)
|
OTVSYK0X
|
ABCA2_HUMAN
|
Decreases Response To Substance
|
[43] |
|
Cyclin-dependent kinase 1 (CDK1)
|
OTW1SC2N
|
CDK1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Serine/threonine-protein kinase 26 (STK26)
|
OTW4QE0D
|
STK26_HUMAN
|
Affects Response To Substance
|
[18] |
|
Homer protein homolog 1 (HOMER1)
|
OTWFD3SI
|
HOME1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Acyl-coenzyme A thioesterase 13 (ACOT13)
|
OTWRUST1
|
ACO13_HUMAN
|
Affects Response To Substance
|
[18] |
|
Divergent protein kinase domain 1A (DIPK1A)
|
OTWS5V2I
|
DIK1A_HUMAN
|
Affects Response To Substance
|
[18] |
|
Urokinase-type plasminogen activator (PLAU)
|
OTX0QGKK
|
UROK_HUMAN
|
Affects Response To Substance
|
[18] |
|
Hematopoietic lineage cell-specific protein (HCLS1)
|
OTX7WGYN
|
HCLS1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Nucleotide triphosphate diphosphatase NUDT15 (NUDT15)
|
OTX8SZOT
|
NUD15_HUMAN
|
Affects Response To Substance
|
[18] |
|
NADH dehydrogenase 1 alpha subcomplex subunit 3 (NDUFA3)
|
OTXL10N9
|
NDUA3_HUMAN
|
Affects Response To Substance
|
[18] |
|
Mitotic spindle assembly checkpoint protein MAD2A (MAD2L1)
|
OTXNGZCG
|
MD2L1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kinesin-like protein KIF20A (KIF20A)
|
OTXOQHE0
|
KI20A_HUMAN
|
Affects Response To Substance
|
[18] |
|
Condensin complex subunit 2 (NCAPH)
|
OTXOS97C
|
CND2_HUMAN
|
Affects Response To Substance
|
[18] |
|
Kinesin-like protein KIF23 (KIF23)
|
OTY850JC
|
KIF23_HUMAN
|
Affects Response To Substance
|
[18] |
|
Protein FAN (NSMAF)
|
OTYNVZ23
|
FAN_HUMAN
|
Affects Response To Substance
|
[18] |
|
Proteasome subunit beta type-1 (PSMB1)
|
OTYRFBAH
|
PSB1_HUMAN
|
Affects Response To Substance
|
[18] |
|
Acetyl-CoA acetyltransferase, cytosolic (ACAT2)
|
OTZ092ZJ
|
THIC_HUMAN
|
Affects Response To Substance
|
[18] |
|
G-protein coupled receptor 176 (GPR176)
|
OTZ8PL9B
|
GP176_HUMAN
|
Affects Response To Substance
|
[18] |
|
Tissue factor pathway inhibitor 2 (TFPI2)
|
OTZCRWOR
|
TFPI2_HUMAN
|
Affects Response To Substance
|
[18] |
|
C-X-C motif chemokine 5 (CXCL5)
|
OTZOUPCA
|
CXCL5_HUMAN
|
Affects Response To Substance
|
[18] |
| ------------------------------------------------------------------------------------ |
|
|
|
|