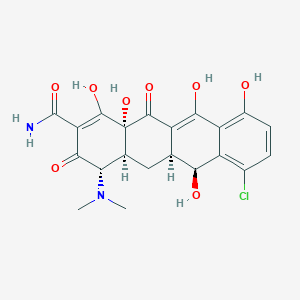
| Synonyms |
Bioterciclin; Clortetrin; DMCT; DMCTC; Declomycin; Deganol; Demeclociclina; Demeclocyclinum; Demeclor; Demethylchlorotetracycline; Demethylchlortetracyclin; Demethylchlortetracycline; Demethylchlortetracyclinum; Demetraclin; Diuciclin; Ledermycin; Methylchlorotetracycline; Mexocine; Novotriclina; Perciclina; Sumaclina; Demeclocycline Monohydrochloride; Demethylchlortetracycline base; Ledermycin hydrochloride; RP 10192; DMCT (antibiotic); Declomycin (TN); Declostatin (TN); Demeclociclina [INN-Spanish]; Demeclocycline (USP); Demeclocycline [USAN:BAN]; Demeclocyclinum [INN-Latin]; Demethylchlortetracycline (JAN); Ledermycin (TN); Tri-demethylchlortetracycline; [4S-(4alpha,4aalpha,5aalpha,6beta,12aalpha)]-7-chloro-4-(dimethylamino)1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide; (2E)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2E,4S,4aS,5aS,6R,12aR)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2E,4S,4aS,5aS,6S,12aS)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z,4S,4aS,5aS,6S,12aS)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (4S,4aS,5aS,6S,12aS)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide; 2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; 6-Demethyl-7-chlorotetracycline; 6-Demethyl-7-chlortetracycline; 6-Demethylchlorotetracycline; 6-Demethylchlortetracycline; 6-Demetil-7-clorotetraciclina; 6-Demetil-7-clorotetraciclina [Italian]; 7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide; 7-Chloro-4-dimethylamino-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic acid amide; 7-Chloro-6-demethyltetracycline
|