Details of the Drug
General Information of Drug (ID: DMZEPFJ)
| Drug Name |
Demeclocycline
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bioterciclin; Clortetrin; DMCT; DMCTC; Declomycin; Deganol; Demeclociclina; Demeclocyclinum; Demeclor; Demethylchlorotetracycline; Demethylchlortetracyclin; Demethylchlortetracycline; Demethylchlortetracyclinum; Demetraclin; Diuciclin; Ledermycin; Methylchlorotetracycline; Mexocine; Novotriclina; Perciclina; Sumaclina; Demeclocycline Monohydrochloride; Demethylchlortetracycline base; Ledermycin hydrochloride; RP 10192; DMCT (antibiotic); Declomycin (TN); Declostatin (TN); Demeclociclina [INN-Spanish]; Demeclocycline (USP); Demeclocycline [USAN:BAN]; Demeclocyclinum [INN-Latin]; Demethylchlortetracycline (JAN); Ledermycin (TN); Tri-demethylchlortetracycline; [4S-(4alpha,4aalpha,5aalpha,6beta,12aalpha)]-7-chloro-4-(dimethylamino)1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide; (2E)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2E,4S,4aS,5aS,6R,12aR)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2E,4S,4aS,5aS,6S,12aS)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z,4S,4aS,5aS,6S,12aS)-2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (4S,4aS,5aS,6S,12aS)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide; 2-[amino(hydroxy)methylidene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; 6-Demethyl-7-chlorotetracycline; 6-Demethyl-7-chlortetracycline; 6-Demethylchlorotetracycline; 6-Demethylchlortetracycline; 6-Demetil-7-clorotetraciclina; 6-Demetil-7-clorotetraciclina [Italian]; 7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-2-naphthacenecarboxamide; 7-Chloro-4-dimethylamino-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic acid amide; 7-Chloro-6-demethyltetracycline
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
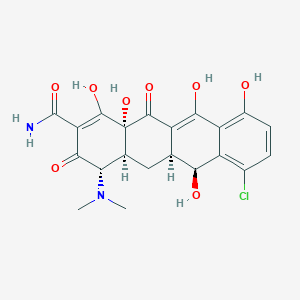 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 464.9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Demeclocycline (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Demeclocycline FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Detection of tetracycline resistance genes by PCR methods. Methods Mol Biol. 2004;268:3-13. | ||||
| 8 | Elliott GR "Sodium bicarbonate and oral tetracycline." Clin Pharmacol Ther 13 (1972): 459. [PMID: 5026384] | ||||
| 9 | Gardner K, Cox T, Digre KB "Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review." Neurology 45 (1995): 6-10. [PMID: 7824136] | ||||
| 10 | Covington TR, Lawson LC, Young LL, eds. "Handbook of Nonprescription Drugs. 10th ed." Washington, DC: American Pharmaceutical Association (1993):. | ||||
| 11 | Gotz VP, Ryerson GG "Evaluation of tetracycline on theophylline disposition in patients with chronic obstructive airways disease." Drug Intell Clin Pharm 20 (1986): 694-6. [PMID: 3757782] | ||||
| 12 | Friedman CI, Huneke AL, Kim MH, Powell J "The effect of ampicillin on oral contraceptive effectiveness." Obstet Gynecol 55 (1980): 33-7. [PMID: 7188714] | ||||
| 13 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 14 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 15 | Lindenbaum J, Rund DG, Butler VP Jr, Tse-Eng D, Saha JR "Inactivation of digoxin by the gut flora: reversal by antibiotic therapy." N Engl J Med 305 (1981): 789-94. [PMID: 7266632] | ||||
| 16 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 17 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 18 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 19 | Campbell NR, Hasinoff BB "Iron supplements: a common cause of drug interactions." Br J Clin Pharmacol 31 (1991): 251-5. [PMID: 2054263] | ||||
| 20 | Blakely KM, Drucker AM, Rosen CF "Drug-induced photosensitivity-an update: Culprit drugs, prevention and management." Drug Saf 42 (2019): 827-47. [PMID: 30888626] | ||||
| 21 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 22 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 23 | Canadian Pharmacists Association. | ||||
| 24 | Albert KS, Welch RD, DeSante KA, DiSanto AR "Decreased tetracycline bioavailability caused by a bismuth subsalicylate antidiarrheal mixture." J Pharm Sci 68 (1979): 586-8. [PMID: 435335] | ||||
