Details of the Drug
General Information of Drug (ID: DMD2NV7)
| Drug Name |
Pindolol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Betapindol; Blocklin; Calvisken; Carvisken; Decreten; Durapindol; Pectobloc; Pinbetol; Pindololum; Prindolol; Prinodolol; Pynastin; Visken; Blockin L; Blocklin L; LB 46; LB46; P 0778; Betapindol (TN); Blockin L (TN); Blocklin L (TN); Blocklin-L; Calvisken (TN); Cardilate (TN); Carvisken (TN); DL-LB 46; DL-Pindolol; Decreten (TN); Durapindol (TN); Glauco-Viskin; Glauco-visken; LB-46; P-6820; Pectobloc (TN); Pinbetol (TN); Pindololum [INN-Latin]; Prindolol (TN); Pynastin (TN); Visken (TN); Blocklin-L (TN); Glauco-Visken (TN); Pindolol (JP15/USP/INN); Pindolol [USAN:INN:BAN:JAN]; DL-4-[2-Hydroxy-3-(isopropylamino)propoxy]indole; (+-)-Pindolol; (1)-1-(1H-Indol-4-yloxy)-3-(isopropylamino)propan-2-ol; 1-((1-Methylethyl)amino)-3-(4-indolyloxy)-2-propanol; 1-(1H-Indol-4-yloxy)-3-((1-methylethyl)amino)-2-propanol; 1-(1H-Indol-4-yloxy)-3-(isopropylamino)-2-propanol; 1-(1H-Indol-4-yloxy)-3-[(1-methylethyl)amino]-2-propanol; 1-(1H-indol-4-yloxy)-3-(isopropylamino)propan-2-ol; 1-(1H-indol-4-yloxy)-3-(propan-2-ylamino)-propan-2-ol; 1-(1H-indol-4-yloxy)-3-(propan-2-ylamino)propan-2-ol; 1-(1H-indol-4-yloxy)-3-[(1-methylethyl)amino]propan-2-ol; 1-(1H-indol-4-yloxy)-3-[(propan-2-yl)amino]propan-2-ol; 1-(Indol-4-yloxy)-3-(isopropylamino)-2-propanol; 4-(2-Hydroxy-3-isopropylaminopropoxy)-indole; 4-(3-(Isopropylamino)-2-hydroxypropoxy)indole
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
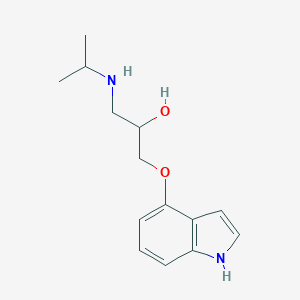 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 248.32 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Pindolol
Coadministration of a Drug Treating the Disease Different from Pindolol (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 91). | ||||
|---|---|---|---|---|---|
| 2 | Health Canada Approved Drug Products: Apo-Pindolol (Pindolol) Oral Tablet | ||||
| 3 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Dailymed: Pindolol Oral Tablet | ||||
| 7 | Are we misunderstanding beta-blockers. Int J Cardiol. 2007 Aug 9;120(1):10-27. | ||||
| 8 | Effects of chronic pindolol treatment on human myocardial beta 1- and beta 2-adrenoceptor function. Naunyn Schmiedebergs Arch Pharmacol. 1990 Oct;342(4):429-35. doi: 10.1007/BF00169460. | ||||
| 9 | Beta-adrenergic receptor blocking drugs, hypertension and plasma renin. Br J Clin Pharmacol. 1975 Apr;2(2):159-64. doi: 10.1111/j.1365-2125.1975.tb01571.x. | ||||
| 10 | Dean S, Kendall MJ, Potter S, Thompson MH, Jackson DA "Nadolol in combination with indapamide and xipamide in resistant hypertensives." Eur J Clin Pharmacol 28 (1985): 29-33. [PMID: 3987783] | ||||
| 11 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 12 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 13 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 14 | Chapple DJ, Clark JS, Hughes R "Interaction between atracurium and drugs used in anaesthesia." Br J Anaesth 55 Suppl 1 (1983): s17-22. [PMID: 6688011] | ||||
| 15 | Canadian Pharmacists Association. | ||||
| 16 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 17 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 18 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Zelboraf (vemurafenib). Genentech, South San Francisco, CA. | ||||
| 21 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 22 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 23 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 24 | Dufresne RL, Weber SS, Becker RE "Bupropion hydrochloride." Drug Intell Clin Pharm 18 (1984): 957-64. [PMID: 6439541] | ||||
| 25 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 26 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
| 27 | Chrysant SG "Experience with terazosin administered in combination with other antihypertensive agents." Am J Med 80 (1986): 55-61. [PMID: 2872808] | ||||
| 28 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 29 | Leor J, Levartowsky D, Sharon C, Farfel Z "Amiodarone and beta-adrenergic blockers: an interaction with metoprolol but not with atenolol." Am Heart J 16 (1988): 206-7. [PMID: 3394625] | ||||
