Details of the Drug
General Information of Drug (ID: DMSVOZT)
| Drug Name |
Nifedipine
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adalat; Adalate; Adapine; Adapress; Afeditab; Alat; Aldipin; Alfadal; Alonix; Angipec; Anifed; Anpine; Aprical; Bonacid; Calcibloc; Calcigard; Calcilat; Camont; Cardifen; Cardilat; Cardionorm; Chronadalate; Citilat; Coracten; Coral; Cordafen; Cordaflex; Cordalat; Cordicant; Cordilan; Cordipin; Cordipine; Corinfar; Corotrend; Corynphar; Depin; Dignokonstant; Dilafed; Dipinkor; Duranifin; Ecodipi; Ecodipin; Emaberin; Fedcor; Fenamon; Fenigidin; Fenihidin; Fenihidine; Glopir; Hadipin; Hexadilat; Infedipin; Introcar; Kordafen; Korinfar; Macorel; Megalat; Myogard; Nedipin; Nicardia; Nifangin; Nifar; Nifdemin; Nifebene; Nifecard; Nifecor; Nifedepat; Nifediac; Nifedical; Nifedicor; Nifedin; Nifedine; Nifedipino; Nifedipinum; Nifedipres; Nifelan; Nifelat; Nifelate; Nificard; Nifidine; Nifipen; Niphedipine; Orix; Oxcord; Pidilat; Procardia; Sepamit; Tibricol; Vascard; Zenusin; AWD Pharma Brand of Nifedipine; Adalat CC; Adalat CR; Adalat Crono; Adalat FT; Adalat GITS; Adalat LA; Adalat LP; Adalat Oros; Adalat PA; Adalat Retard; Adalat XL; Adalate LP; Adcock Ingram Brand of Nifedipine; Adipine XL; Afeditab CR; Alonix S; Aprical long; Bayer Brand of Nifedipine; Chronadalate LP; Coracten XL; Ecodipin E; Fedcor Retard; Fenamon SR; Fortipine LA; KRKA Brand of Nifedipine; Nifedical XL; Nifedipine Bayer Brand; Nifedipine GTIS; Nifedipine KRKA Brand; Nifedipine Monohydrochloride; Nifedipine Orion Brand; Nifedipine Pfizer Brand; Nifedipine Retard; Nifedirex LP; Nifelat Q; Nifensar XL; Orion Brand of Nifedipine; Pfizer Brand of Nifedipine; Procardia XL; Slofedipine XL; Tensipine MR; Adalat 10; Adalat 20; Adalat 5; Adalat GITS 30; Bay1040; N 7634; N1fedilat; Adalat (TN); Afeditab CR (TN); Alpha-Nifedipine Retard;Apo-Nifed; Bay-1040; KB-1712P; Monohydrochloride, Nifedipine; Nifedical (TN); Nifedipine-GTIS; Nifedipino [INN-Spanish]; Nifedipinum [INN-Latin]; Procardia (TN); Bay-a-1040; Nifedipine (JP15/USP/INN); Nifedipine [USAN:BAN:INN:JAN]
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
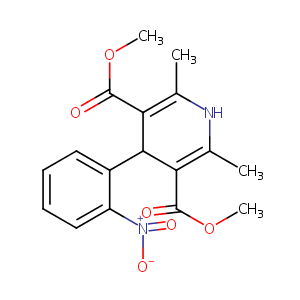 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 346.3 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimental Cancer Drug Sensitivity Information
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Nifedipine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
| DIG |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmaceutical Formulation |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2514). | ||||
|---|---|---|---|---|---|
| 2 | Nifedipine FDA Label | ||||
| 3 | van Harten J, Burggraaf K, Danhof M, van Brummelen P, Breimer DD: Negligible sublingual absorption of nifedipine. Lancet. 1987 Dec 12;2(8572):1363-5. doi: 10.1016/s0140-6736(87)91258-x. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Clinical pharmacokinetics of nifedipine gastrointestinal therapeutic system. A controlled-release formulation of nifedipine. Am J Med. 1987 Dec 21;83(6B):10-4. doi: 10.1016/0002-9343(87)90630-9. | ||||
| 6 | FDA Approved Drug Products: Procardia XL Nifedipine Oral Extended Release Tablets | ||||
| 7 | Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W: The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008 Feb;36(2):386-99. doi: 10.1124/dmd.107.019083. Epub 2007 Nov 15. | ||||
| 8 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 9 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 10 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 11 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 12 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | ||||
| 13 | Metabolic interactions of selected antimalarial and non-antimalarial drugs with the major pathway (3-hydroxylation) of quinine in human liver microsomes. Br J Clin Pharmacol. 1997 Nov;44(5):505-11. | ||||
| 14 | Molecular basis of polymorphic drug metabolism. J Mol Med (Berl). 1995 Nov;73(11):539-53. | ||||
| 15 | Inhibition of human cytochrome P450 enzymes by 1,4-dihydropyridine calcium antagonists: prediction of in vivo drug-drug interactions. Eur J Clin Pharmacol. 2000 Feb-Mar;55(11-12):843-52. | ||||
| 16 | In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes p450: role of cyp3a4 and cyp3a5. Drug Metab Dispos. 2003 Jul;31(7):938-44. | ||||
| 17 | Wild-type CYP102A1 as a biocatalyst: turnover of drugs usually metabolised by human liver enzymes. J Biol Inorg Chem. 2007 Mar;12(3):313-23. | ||||
| 18 | The bacterial P450 BM3: a prototype for a biocatalyst with human P450 activities. Trends Biotechnol. 2007 Jul;25(7):289-98. | ||||
| 19 | Effects of nifedipine GITS and atenolol monotherapy on serum lipids, blood pressure, heart rate, and weight in mild to moderate hypertension. Angiology. 1991 Sep;42(9):681-90. doi: 10.1177/000331979104200901. | ||||
| 20 | Reduced bcl-2 concentrations in hypertensive patients after lisinopril or nifedipine administration. Am J Hypertens. 1999 Jan;12(1 Pt 1):73-5. doi: 10.1016/s0895-7061(98)00217-9. | ||||
| 21 | Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos. 2003 Nov;31(11):1296-9. doi: 10.1124/dmd.31.11.1296. | ||||
| 22 | Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996 Feb;49(2):311-8. | ||||
| 23 | Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Sci Rep. 2014 Sep 26;4:6437. doi: 10.1038/srep06437. | ||||
| 24 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | ||||
| 25 | High-throughput measurement of the Tp53 response to anticancer drugs and random compounds using a stably integrated Tp53-responsive luciferase reporter. Carcinogenesis. 2002 Jun;23(6):949-57. doi: 10.1093/carcin/23.6.949. | ||||
| 26 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 27 | Product Information. Actos (pioglitazone) Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 28 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 29 | Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y "Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers." Cancer Chemother Pharmacol 72 (2013): 1223-34. [PMID: 24085261] | ||||
| 30 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 31 | Product Information. Mycobutin (rifabutin). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 32 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 33 | Hedaya MA, El-Afify DR, El-Maghraby GM "The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers." Biopharm Drug Dispos 27 (2006): 103-10. [PMID: 16372380] | ||||
| 34 | Heinig R, Adelmann HG, Ahr G "The effect of ketoconazole on the pharmacokinetics, pharmacodynamics and safety of nisoldipine." Eur J Clin Pharmacol 55 (1999): 57-60. [PMID: 10206086] | ||||
| 35 | Bolland MJ, Bagg W, Thomas MG, Lucas JA, Ticehurst R, Black PN "Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole." Ann Pharmacother 38 (2004): 46-9. [PMID: 14742792] | ||||
| 36 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 37 | Geronimo-Pardo M, Cuartero-Del-Pozo AB, Jimenez-Vizuete JM, Cortinas-Saez M, Peyro-Garcia R "Clarithromycin-nifedipine interaction as possible cause of vasodilatory shock." Ann Pharmacother 39 (2005): 538-42. [PMID: 15703161] | ||||
| 38 | Product Information. Vraylar (cariprazine). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 39 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 40 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 41 | Product Information. Talzenna (talazoparib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 42 | Product Information. Ixempra (ixabepilone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 43 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 44 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 45 | Product Information. Jevtana (cabazitaxel). sanofi-aventis , Bridgewater, NJ. | ||||
| 46 | Product Information. Opsumit (macitentan). Actelion Pharmaceuticals US Inc, South San Francisco, CA. | ||||
| 47 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 48 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 49 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 50 | Product Information. Ofev (nintedanib). Boehringer Ingelheim, Ridgefield, CT. | ||||
| 51 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 52 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 53 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 54 | Product Information. Serzone (nefazodone). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 55 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 56 | Product Information. Celexa (citalopram). Forest Pharmaceuticals, St. Louis, MO. | ||||
| 57 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 58 | Product Information. Polivy (polatuzumab vedotin). Genentech, South San Francisco, CA. | ||||
| 59 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 60 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 61 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 62 | Product Information. VESIcare (solifenacin). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 63 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 64 | Henry M, Kay MM, Viccellio P "Cardiogenic shock associated with calcium-channel and beta blockers: reversal with intravenous calcium chloride." Am J Emerg Med 3 (1985): 334-6. [PMID: 2860911] | ||||
| 65 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 66 | Product Information. Olysio (simeprevir). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 67 | Product Information. Pifeltro (doravirine). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 68 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 69 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 70 | Product Information. Tivicay (dolutegravir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 71 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 72 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 73 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 74 | Product Information. Selzentry (maraviroc). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 75 | Canadian Pharmacists Association. | ||||
| 76 | Product Information. Samsca (tolvaptan). Otsuka American Pharmaceuticals Inc, Rockville, MD. | ||||
| 77 | Product Information. Altabax (retapamulin topical). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 78 | Product Information. Zurampic (lesinurad). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 79 | Hamann SR, Blouin RA, Chang SL, et al "Effects of hemodynamic changes on the elimination kinetics of verapamil and nifedipine." J Pharmacol Exp Ther 231 (1984): 301-5. [PMID: 6491984] | ||||
| 80 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 81 | Product Information. Movantik (naloxegol). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 82 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 83 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 84 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 85 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 86 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 87 | Product Information. Imbruvica (ibrutinib). Pharmacyclics Inc, Sunnyvale, CA. | ||||
| 88 | Product Information. Koselugo (selumetinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 89 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 90 | Product Information. Ubrelvy (ubrogepant). Allergan Inc, Irvine, CA. | ||||
| 91 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 92 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 93 | Hamberg P, Woo MM, Chen LC, et al. "Effect of ketoconazole-mediated CYP3A4 inhibition on clinical pharmacokinetics of panobinostat (LBH589), an orally active histone deacetylase inhibitor." Cancer Chemother Pharmacol 68 (2011): 805-13. [PMID: 21706316] | ||||
| 94 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 95 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 96 | Product Information. Istodax (romidepsin). Gloucester Pharmaceuticals, Cambridge, MA. | ||||
| 97 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 98 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 99 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 100 | Product Information. Lynparza (olaparib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 101 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 102 | Product Information. Nourianz (istradefylline). Kyowa Kirin, Inc, Bedminster, NJ. | ||||
| 103 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 104 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 105 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 106 | Francis H, Tyndall A, Webb J "Severe vascular spasm due to erythromycin-ergotamine interaction." Clin Rheumatol 3 (1984): 243-6. [PMID: 6236021] | ||||
| 107 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 108 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 109 | Product Information. Duagen (dutasteride). GlaxoSmithKline Healthcare, Pittsburgh, PA. | ||||
| 110 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 111 | Product Information. Rinvoq (upadacitinib). AbbVie US LLC, North Chicago, IL. | ||||
| 112 | DeVane CL, Nemeroff CB "Clinical pharmacokinetics of quetiapine - An atypical antipsychotic." Clin Pharmacokinet 40 (2001): 509-22. [PMID: 11510628] | ||||
| 113 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 114 | Product Information. Odomzo (sonidegib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 115 | Aronson JK, Grahame-Smith DG "Clinical pharmacology: adverse drug interactions." Br Med J 282 (1981): 288-91. [PMID: 6779990] | ||||
| 116 | Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K "Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60 year old Caucasian woman with ovarian carcinoma." Br J Clin Pharmacol (2015):. [PMID: 26446447] | ||||
| 117 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 118 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 119 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
