Details of the Drug
General Information of Drug (ID: DM2POTE)
| Drug Name |
Colchicine
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Colchicin; Colchicina; Colchicinum; Colchineos; Colchisol; Colchysat; Colcin; Colcrys; Colsaloid; Colstat; Condylon; Goutnil; Kolkicin; LOC; Binds to tubulin; Colchicin [German]; Colchicina [Italian]; Colchicine [JAN]; Inhibits microtubular assembly; Spindle poison; C 9754; Colchicine (TN); Colchicine, Colchicum autumnale; MPC-004; N-Acetyl trimethylcolchicinic acid methylether; Colchicine (JP15/USP); Colchicine, (R)-Isomer; Benzo(a)heptalen-9(5H)-one; Colchicine, (+-)-Isomer; N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide; N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide; N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide; N-[(7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl]acetamide; N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[.alpha.]heptalen-7-yl)-acetamide; N-((7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)-acetamide; (S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl)acetamide; 7-alpha-H-Colchicine; 7.alpha.H-Colchicine; 7alphaH-Colchicine
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Gout Suppressants
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
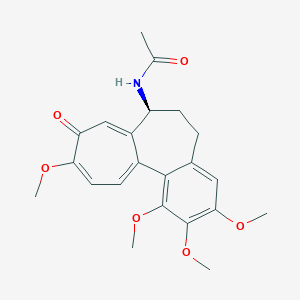 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 399.4 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Acute gout flare | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | FA25.0 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Colchicine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 022351 | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7526). | ||||
| 3 | Colchicine FDA Label | ||||
| 4 | ClinicalTrials.gov (NCT04322565) Colchicine Counteracting Inflammation in COVID-19 Pneumonia. U.S. National Institutes of Health. | ||||
| 5 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 6 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Vitamin K3 disrupts the microtubule networks by binding to tubulin: a novel mechanism of its antiproliferative activity. Biochemistry. 2009 Jul 28;48(29):6963-74. | ||||
| 9 | Pharmacological characterization of the murine and human orthologs of multidrug-resistance protein in transfected human embryonic kidney cells. Mol Pharmacol. 1997 Sep;52(3):344-53. | ||||
| 10 | Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol. 2009 Jul 15;78(2):153-61. | ||||
| 11 | Colchicine down-regulates cytochrome P450 2B6, 2C8, 2C9, and 3A4 in human hepatocytes by affecting their glucocorticoid receptor-mediated regulation. Mol Pharmacol. 2003 Jul;64(1):160-9. | ||||
| 12 | Utilization of CDKN1A/p21 gene for class discrimination of DNA damage-induced clastogenicity. Toxicology. 2014 Jan 6;315:8-16. doi: 10.1016/j.tox.2013.10.009. Epub 2013 Nov 6. | ||||
| 13 | Dihydroactinidiolide regulates Nrf2/HO-1 expression and inhibits caspase-3/Bax pathway to protect SH-SY5Y human neuroblastoma cells from oxidative stress induced neuronal apoptosis. Neurotoxicology. 2021 May;84:53-63. doi: 10.1016/j.neuro.2021.02.006. Epub 2021 Feb 20. | ||||
| 14 | [Colchicine in chronic liver disease of alcoholic etiology. Double-blind, randomized study of its effects on blood levels of plasma proteins and clinical course in patients]. Rev Assoc Med Bras (1992). 1995 May-Jun;41(3):207-12. | ||||
| 15 | Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17. | ||||
| 16 | Colchicine-induced apoptosis in human normal liver L-02 cells by mitochondrial mediated pathways. Toxicol In Vitro. 2012 Aug;26(5):649-55. doi: 10.1016/j.tiv.2012.01.024. Epub 2012 Feb 8. | ||||
| 17 | Involvement of cytoskeleton in AhR-dependent CYP1A1 expression. Curr Drug Metab. 2006 Apr;7(3):301-13. doi: 10.2174/138920006776359310. | ||||
| 18 | Population-based in vitro hazard and concentration-response assessment of chemicals: the 1000 genomes high-throughput screening study. Environ Health Perspect. 2015 May;123(5):458-66. doi: 10.1289/ehp.1408775. Epub 2015 Jan 13. | ||||
| 19 | Analysis of ATP-binding cassette transporter expression in drug-selected cell lines by a microarray dedicated to multidrug resistance. Mol Pharmacol. 2004 Dec;66(6):1397-405. doi: 10.1124/mol.104.005009. Epub 2004 Sep 1. | ||||
| 20 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 21 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 22 | Akdag I, Ersoy A, Kahvecioglu S, Gullulu M, Dilek K "Acute colchicine intoxication during clarithromycin administration in patients with chronic renal failure." J Nephrol 19 (2006): 515-7. [PMID: 17048210] | ||||
| 23 | Dogukan A, Oymak FS, Taskapan H, Guven M, Tokgoz B, Utas C "Acute fatal colchicine intoxication in a patient on continuous ambulatory peritoneal dialysis (CAPD). Possible role of clarithromycin administration." Clin Nephrol 55 (2001): 181-2. [PMID: 11269688] | ||||
| 24 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 25 | Product Information. Ibrance (palbociclib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 26 | Carrion C, Espinosa E, Herrero A, Garcia B "Possible vincristine-isoniazid interaction." Ann Pharmacother 29 (1995): 201. [PMID: 7756727] | ||||
| 27 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 28 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 29 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 30 | Product Information. Blincyto (blinatumomab). Amgen USA, Thousand Oaks, CA. | ||||
| 31 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 32 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 33 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 34 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 35 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 36 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 37 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 38 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 39 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
