Details of the Drug
General Information of Drug (ID: DMKLAYG)
| Drug Name |
Altretamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Altretamina; Altretaminum; HEXAMETHYLMELAMINE; HMM; HTM; HXM; Hemel; Hexalen; Hexastat; Hexinawas; Altretamine Bellon Brand; Altretamine Chiesi Brand; Altretamine Wassermann Brand; Bellon Brand of Altretamine; Chiesi Brand of Altretamine; MGI Pharma Brand of Altretamine; Rhone Poulenc Rorer Brand of Altretamine; Wassermann Brand of Altretamine; A 8723; ENT 50852; NC 195; Altretamina [INN-Spanish]; Altretaminum [INN-Latin]; Hexalen (TN); Hexalen, Altretamine; KB-913; Rhone-Poulenc Rorer Brand of Altretamine; Altretamine (USP/INN); Altretamine [USAN:INN:BAN]; No-s-triazine; N,N,N',N',N'',N''-hexamethyl-1,3,5-triazine-2,4,6-triamine; N~2~,N~2~,N~4~,N~4~,N~6~,N~6~-Hexamethyl-1,3,5-triazine-2,4,6-triamine; 2,4, 6-Tris(dimethylamino)-1,3,5-triazine; 2,4,6-Tris(dimethylami; 2,4,6-Tris(dimethylamino)-1,3,5-triazine; 2,4,6-Tris(dimethylamino-s-triazine; 2-N,2-N,4-N,4-N,6-N,6-N-hexamethyl-1,3,5-triazine-2,4,6-triamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
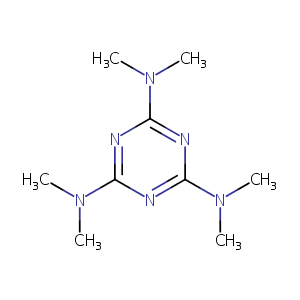 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 210.28 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Altretamine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7112). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Synergy of irofulven in combination with other DNA damaging agents: synergistic interaction with altretamine, alkylating, and platinum-derived agen... Cancer Chemother Pharmacol. 2008 Dec;63(1):19-26. | ||||
| 6 | Paolini A, D'Incalci M "Effect of phenobarbital pretreatment on the metabolism and antitumor activity of hexamethylmelamine." Cancer Treat Rep 70 (1986): 513-6. [PMID: 3084083] | ||||
| 7 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 8 | Bruckner HW, Schleifer SJ "Orthostatic hypotension as a complication of hexamethylmelamine antidepressant interaction." Cancer Treat Rep 67 (1983): 516. [PMID: 6406063] | ||||
| 9 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 10 | Hande K, Combs G, Swingle R, Combs GL, Anthony L "Effect of cimetidine and ranitidine on the metabolism and toxicity of hexamethylmelamine." Cancer Treat Rep 70 (1986): 1443-5. [PMID: 3098418] | ||||
| 11 | Product Information. Arava (leflunomide). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 12 | Product Information. Prolia (denosumab). Amgen USA, Thousand Oaks, CA. | ||||
| 13 | Figg WD, Arlen P, Gulley J, et al. "A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer." Semin Oncol 28(4 Suppl 15) (2001): 62-6. [PMID: 11685731] | ||||
| 14 | Product Information. Vumerity (diroximel fumarate). Alkermes, Inc, Cambridge, MA. | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 17 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 18 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 19 | Product Information. Arcalyst (rilonacept). Regeneron Pharmaceuticals Inc, Tarrytown, NY. | ||||
| 20 | Product Information. Cimzia (certolizumab). UCB Pharma Inc, Smyrna, GA. | ||||
| 21 | CDC. Centers for Disease Control and Prevention/ "Recommendations of the advisory committtee on immunization practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence." MMWR Morb Mortal Wkly Rep 42(RR-04) (1993): 1-18. [PMID: 20300058] | ||||
