Details of the Drug
General Information of Drug (ID: DM4EWNS)
| Drug Name |
Cinoxacin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cinobac; Cinobactin; Cinoxacine; Cinoxacino; Cinoxacinum; Cinx; Clinoxacin; Uronorm; Azolinic Acid; C 8645; Lilly 64716; TNP00246; Cinobac (TN); Cinoxacine [INN-French]; Cinoxacino [INN-Spanish]; Cinoxacinum [INN-Latin]; Cinoxacin (JAN/USP/INN); Cinoxacin [USAN:BAN:INN:JAN]; 1-Ethyl-1,4-dihydro-4-oxo(1,3)dioxolo(4,5-g)cinnoline-3-carboxylic acid; 1-Ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; 1-Ethyl-4-oxo-1,4-dihydro[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid; 1-Ethyl-6,7-methylenedioxy-4(1H)-oxocinnoline-3-carboxylic acid; 1-ethyl-4-oxo-[1,3]dioxolo[4,5-g]cinnoline-3-carboxylic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
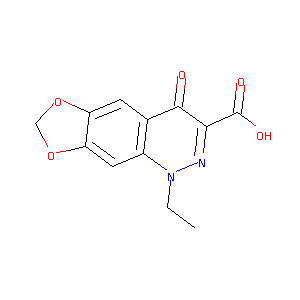 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 262.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Cinoxacin (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | The interaction and transport of beta-lactam antibiotics with the cloned rat renal organic anion transporter 1. J Pharmacol Exp Ther. 1999 Aug;290(2):672-7. | ||||
| 7 | Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3. | ||||
| 8 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 9 | Ball P "Ciprofloxacin: an overview of adverse experiences." J Antimicrob Chemother 18 (1986): 187-93. [PMID: 3542945] | ||||
| 10 | Product Information. Factive (gemifloxacin). GeneSoft Inc, San Francisco, CA. | ||||
| 11 | Friedman CI, Huneke AL, Kim MH, Powell J "The effect of ampicillin on oral contraceptive effectiveness." Obstet Gynecol 55 (1980): 33-7. [PMID: 7188714] | ||||
| 12 | Product Information. CellCept (mycophenolate mofetil). Roche Laboratories, Nutley, NJ. | ||||
| 13 | Deppermann KM, Lode H "Fluoroquinolones: interaction profile during enteral absorption." Drugs 45 Suppl 3 (1993): 65-72. [PMID: 7689454] | ||||
| 14 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
| 15 | Davey PG "Overview of drug interactions with the quinolones." J Antimicrob Chemother 22(suppl c) (1988): 97-107. [PMID: 3053579] | ||||
| 16 | Nix DE, Wilton JH, Ronald B, Distlerath L, Williams VC, Norman A "Inhibition of norfloxacin absorption by antacids." Antimicrob Agents Chemother 34 (1990): 432-5. [PMID: 2334155] | ||||
| 17 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 18 | Canadian Pharmacists Association. | ||||
| 19 | FDA. U.S. Food and Drug Administration "Information for Healthcare Professionals. Fluoroquinolone Antimicrobial Drugs. FDA Alert [7/8/2008].". | ||||
| 20 | Product Information. Wellbutrin XL (buPROPion). GlaxoSmithKline, Philadelphia, PA. | ||||
| 21 | Product Information. Avelox (moxifloxacin) Bayer, West Haven, CT. | ||||
