Details of the Drug
General Information of Drug (ID: DM4KDYJ)
| Drug Name |
Nortriptyline
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acetexa; Allegron; Altilev; Ateben; Avantyl; Aventyl; Demethylamitriptylene; Demethylamitriptyline; Demethylamitryptyline; Desitriptilina; Desitriptyline; Desmethylamitriptylin; Desmethylamitriptyline; Lumbeck; Noramitriptyline; Noritren; Nortrilen; Nortriptilina; Nortriptylinum; Nortryptiline; Nortryptyline; Pamelor; Psychostyl; Sensaval; Sesaval; Vividyl; AVENTYL HCL; Nortriptilina [DCIT]; Nortriptylene hydrochloride; Pamelor hydrochloride; Sensival Ventyl; NCI169453; Allegron (TN); Aventyl (TN); Norpress (TN); Nortrilen (TN); Nortriptyline (INN); Nortriptyline [INN:BAN]; Nortriptylinum [INN-Latin]; Pamelor (TN); Sensoval (TN); N-Methyl-3-(10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5-yliden)propylamin; (2)10,11-Dihydro-N-methyl-5H-dibenzo[a,d]cycloheptene-.delta.5.gamma.-propylamine; 10,11-Dihydro-5-(3-methylaminopropylidene)-5H-dibenzo(a,d)(1,4)cycloheptene; 10,11-Dihydro-N-methyl-5H-dibenzo(a,d)cycloheptene-delta(5,gamma)-propylamine; 10,11-dihydro-N-methyl-5H-dibenzo[a,d]cycloheptene-Delta(5,gamma)-propylamine; 3-(10,11-Dihydro-5H-dibenzo(a,d)cyclohepten-5-ylidene)-N-methylpropylamine; 3-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N-methyl-1-propanamine; 3-(10,11-dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-N-methylpropan-1-amine; 5-(3-(Methylamino)propylidene)dibenzo(a,e)cyclohepta(1,5)diene; 5-(3-Methylaminopropylidene)-10,11-dihydro-5H-dibenzo(a,d)cycloheptene; 5-(alpha-Methylaminopropylidene)dibenzo(a,d)cyclohepta(1,4)diene
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antidepressants
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
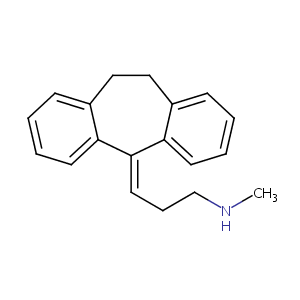 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 263.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Depression | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A70-6A7Z | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Nortriptyline
Coadministration of a Drug Treating the Disease Different from Nortriptyline (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2404). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Dawling S, Crome P, Braithwaite R: Pharmacokinetics of single oral doses of nortriptyline in depressed elderly hospital patients and young healthy volunteers. Clin Pharmacokinet. 1980 Jul-Aug;5(4):394-401. doi: 10.2165/00003088-198005040-00007. | ||||
| 4 | MedSafe NZ data sheet: Nortriptyline tablets | ||||
| 5 | Pharmacokinetic aspects on once-daily nortriptyline administration. Neuropsychobiology. 1980;6(1):34-41. doi: 10.1159/000117730. | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 9 | Cytochrome P-450 activities in human and rat brain microsomes. Brain Res. 2000 Feb 14;855(2):235-43. | ||||
| 10 | A study on CYP2C19 and CYP2D6 polymorphic effects on pharmacokinetics and pharmacodynamics of amitriptyline in healthy Koreans. Clin Transl Sci. 2017 Mar;10(2):93-101. | ||||
| 11 | In vivo age-related changes in hepatic drug-oxidizing capacity in humans. J Clin Pharm Ther. 1998 Aug;23(4):247-55. | ||||
| 12 | Hydroxylation and demethylation of the tricyclic antidepressant nortriptyline by cDNA-expressed human cytochrome P-450 isozymes. Drug Metab Dispos. 1997 Jun;25(6):740-4. | ||||
| 13 | Bioactivation of the tricyclic antidepressant amitriptyline and its metabolite nortriptyline to arene oxide intermediates in human liver microsomes and recombinant P450s. Chem Biol Interact. 2008 May 9;173(1):59-67. | ||||
| 14 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 15 | Downregulation of serotonin receptor subtypes by nortriptyline and adinazolam in major depressive disorder: neuroendocrine and platelet markers. Clin Neuropharmacol. 1993;16 Suppl 3:S19-31. | ||||
| 16 | A common P-glycoprotein polymorphism is associated with nortriptyline-induced postural hypotension in patients treated for major depression. Pharmacogenomics J. 2002;2(3):191-6. | ||||
| 17 | Palmitate increases the susceptibility of cells to drug-induced toxicity: an in vitro method to identify drugs with potential contraindications in patients with metabolic disease. Toxicol Sci. 2012 Oct;129(2):346-62. doi: 10.1093/toxsci/kfs208. Epub 2012 Jun 14. | ||||
| 18 | Mechanism-based inactivation of human cytochrome P4502C8 by drugs in vitro. J Pharmacol Exp Ther. 2004 Dec;311(3):996-1007. | ||||
| 19 | Inhibition of cytochrome P4502D6 activity with paroxetine normalizes the ultrarapid metabolizer phenotype as measured by nortriptyline pharmacokinetics and the debrisoquin test. Clin Pharmacol Ther. 2001 Oct;70(4):327-35. | ||||
| 20 | Age-dependent antidepressant pharmacogenomics: polymorphisms of the serotonin transporter and G protein beta3 subunit as predictors of response to fluoxetine and nortriptyline. Int J Neuropsychopharmacol. 2003 Dec;6(4):339-46. doi: 10.1017/S1461145703003663. | ||||
| 21 | Cohen MA, Alfonso CA, Mosquera M. Development of urinary retention during treatment with clozapine and meclizine [published correction appears in Am J Psychiatry 1994 Jun;151(6):952]. Am J Psychiatry. 1994;151(4):619-620. [PMID: 8147469] | ||||
| 22 | Achamallah NS "Visual hallucinations after combining fluoxetine and dextromethorphan ." Am J Psychiatry 149 (1992): 1406. [PMID: 1530079] | ||||
| 23 | Albers LJ, Reist C, Helmeste D, Vu R, Tang SW "Paroxetine shifts imipramine metabolism." Psychiatry Res 59 (1996): 189-96. [PMID: 8930024] | ||||
| 24 | Boyer EW, Shannon M "The serotonin syndrome." N Engl J Med 352 (2005): 1112-20. [PMID: 15784664] | ||||
| 25 | Bhatara VS, Magnus RD, Paul KL, Preskorn SH "Serotonin syndrome induced by venlafaxine and fluoxetine: a case study in polypharmacy and potential pharmacodynamic and pharmacokinetic mechanisms." Ann Pharmacother 32 (1998): 432-6. [PMID: 9562139] | ||||
| 26 | Kulik AV, Wilbur R "Delirium and stereotypy from anticholinergic antiparkinson drugs." Prog Neuropsychopharmacol Biol Psychiatry 6 (1982): 75-82. [PMID: 7202232] | ||||
| 27 | Product Information. Tibsovo (ivosidenib). Agios Pharmaceuticals, Cambridge, MA. | ||||
| 28 | Canadian Pharmacists Association. | ||||
| 29 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 30 | Product Information. Xospata (gilteritinib). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 31 | Vizcaychipi MP, Walker S, Palazzo M "Serotonin syndrome triggered by tramadol." Br J Anaesth 99 (2007): 919. [PMID: 18006535] | ||||
| 32 | Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC "Explicit criteria for determining inappropriate medication use in nursing home residents." Arch Intern Med 151 (1991): 1825-32. [PMID: 1888249] | ||||
| 33 | Hollingshead LM, Faulds D, Fitton A "Bepridil. A review of its pharmacological properties and therapeutic use in stable angina pectoris." Drugs 44 (1992): 835-57. [PMID: 1280569] | ||||
| 34 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 35 | Ball P "Quinolone-induced QT interval prolongation: a not-so-unexpected class effect." J Antimicrob Chemother 45 (2000): 557-9. [PMID: 10797074] | ||||
| 36 | Bergeron L, Boule M, Perreault S "Serotonin toxicity associated with concomitant use of linezolid." Ann Pharmacother 39 (2005): 956-61. [PMID: 15827071] | ||||
| 37 | Eronen M, Putkonen H, Hallikainen T, Vartiainen H "Lethal gastroenteritis associated with clozapine and loperamide." Am J Psychiatry 160 (2003): 2242-2243. [PMID: 14638602] | ||||
| 38 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 39 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 40 | Cole JM, Sheehan AH, Jordan JK "Concomitant use of ipratropium and tiotropium in chronic obstructive plmonary disease." Ann Pharmacother 46 (2012): 1717-21. [PMID: 23170031] | ||||
| 41 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 42 | Edelbroek PM, Zitman FG, Knoppert-van der Klein EA, van Putten PM, de Wolff FA "Therapeutic drug monitoring of amitriptyline: impact of age, smoking and contraceptives on drug and metabolite levels in bulimic women." Clin Chim Acta 165 (1987): 177-87. [PMID: 3652444] | ||||
| 43 | Product Information. Lamprene (clofazimine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 44 | Ciraulo DA, Alderson LM, Chapron DJ, Jaffe JH, Subbarao B, Kramer PA "Imipramine disposition in alcoholics." J Clin Psychopharmacol 2 (1982): 2-7. [PMID: 7068940] | ||||
| 45 | Ciraulo DA, Shader RI "Fluoxetine drug-drug interactions: I. Antidepressants and antipsychotics." J Clin Psychopharmacol 10 (1990): 48-50. [PMID: 1968472] | ||||
| 46 | Product Information. Austedo (deutetrabenazine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 47 | Product Information. Ingrezza (valbenazine). Neurocrine Biosciences, Inc., San Diego, CA. | ||||
| 48 | Product Information. Zonegran (zonisamide) Elan Pharmaceuticals, S. San Francisco, CA. | ||||
| 49 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 50 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 51 | Brosen K, Kragh-Sorensen P "Concomitant intake of nortriptyline and carbamazepine." Ther Drug Monit 15 (1993): 258-60. [PMID: 8333008] | ||||
| 52 | Product Information. Myrbetriq (mirabegron). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 53 | AbdelRahman SM, Gotschall RR, Kauffman RE, Leeder JS, Kearns GL "Investigation of terbinafine as a CYP2D6 inhibitor in vivo." Clin Pharmacol Ther 65 (1999): 465-72. [PMID: 10340911] | ||||
| 54 | Dorsey ST, Biblio LA "Prolonged QT interval and torsades de pointes caused by the combination of fluconazole and amitriptyline." Am J Emerg Med 18 (2000): 227-9. [PMID: 10750939] | ||||
| 55 | Gram LF, Kofod B, Christiansen J, Rafaelsen OJ "Imipramine metabolism: pH-dependent distribution and urinary excretion." Clin Pharmacol Ther 12 (1971): 239-44. [PMID: 5554940] | ||||
| 56 | Product Information. Rukobia (fostemsavir). ViiV Healthcare, Research Triangle Park, NC. | ||||
| 57 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 58 | Product Information. Sustiva (efavirenz). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 59 | Anson BD, Weaver JG, Ackerman MJ, et al. "Blockade of HERG channels by HIV protease inhibitors." Lancet 365 (2005): 682-686. [PMID: 15721475] | ||||
| 60 | Hermann DJ, Krol TF, Dukes GE, et al "Comparison of verapamil, diltiazem, and labetalol on the bioavailability and metabolism of imipramine." J Clin Pharmacol 32 (1992): 176-83. [PMID: 1613128] | ||||
| 61 | Product Information. Givlaari (givosiran). Alnylam Pharmaceuticals, Cambridge, MA. | ||||
| 62 | Postma JU, van Tilburg W "Visual hallucinations and delirium during treatment with amantadine (Symmetrel)." J Am Geriatr Soc 23 (1975): 212-5. [PMID: 123540] | ||||
| 63 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 64 | Bock JL, Nelson JC, Gray S, Jatlow PI "Desipramine hydroxylation: variability and effect of antipsychotic drugs." Clin Pharmacol Ther 33 (1983): 322-8. [PMID: 6130865] | ||||
| 65 | Product Information. Xalkori (crizotinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 66 | Blakely KM, Drucker AM, Rosen CF "Drug-induced photosensitivity-an update: Culprit drugs, prevention and management." Drug Saf 42 (2019): 827-47. [PMID: 30888626] | ||||
| 67 | Product Information. Vizimpro (dacomitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 68 | Product Information. Tagrisso (osimertinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 69 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 70 | Abernethy DR, Wesche DL, Barbey JT, et al. "Stereoselective halofantrine disposition and effect: concentration-related QTc prolongation." Br J Clin Pharmacol 51 (2001): 231-7. [PMID: 11298069] | ||||
| 71 | Harper KM, Knapp DJ, Criswell HE, Breese GR "Vasopressin and alcohol: A multifaceted relationship." Psychopharmacology (Berl) 235 (2018): 3363-79. [PMID: 32936259] | ||||
| 72 | Ohnishi K, Yoshida H, Shigeno K, et al. "Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia." Ann Intern Med 133 (2000): 881-5. [PMID: 11103058] | ||||
| 73 | Product Information. Braftovi (encorafenib). Array BioPharma Inc., Boulder, CO. | ||||
| 74 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 75 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 76 | Product Information. Farydak (panobinostat). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 77 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 78 | Alvine G, Black DW, Tsuang D "Case of delirium secondary to phenelzine/L-tryptophan combination." J Clin Psychiatry 51 (1990): 311. [PMID: 2365671] | ||||
| 79 | Product Information. Gleevec (imatinib mesylate). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 80 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 81 | Product Information. Varubi (rolapitant). Tesaro Inc., Waltham, MA. | ||||
| 82 | Chan BSH, Graudins A, Whyte IM, Dawson AH, Braitberg G, Duggin GG "Serotonin syndrome resulting from drug interactions." Med J Aust 169 (1998): 523-5. [PMID: 9861909] | ||||
| 83 | Product Information. Belviq (lorcaserin). Eisai Inc, Teaneck, NJ. | ||||
| 84 | Product Information. Iopidine (apraclonidine). Alcon Laboratories Inc, Fort Worth, TX. | ||||
| 85 | Abernethy DR, Greenblatt DJ, Steel K, Shader RI "Impairment of hepatic drug oxidation by propoxyphene." Ann Intern Med 97 (1982): 223-4. [PMID: 7103282] | ||||
| 86 | Morgan JP, Rivera-Calimlim L, Messiha F, Sundaresan PR, Trabert N "Imipramine-mediated interference with levodopa absorption from the gastrointestinal tract in man." Neurology 25 (1975): 1029-34. [PMID: 1237820] | ||||
| 87 | Product Information. Nuplazid (pimavanserin). Accelis Pharma, East Windsor, NJ. | ||||
| 88 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 89 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 90 | Product Information. Zytiga (abiraterone). Centocor Inc, Malvern, PA. | ||||
| 91 | Ansari SR, Chopra N "Gatifloxacin and Prolonged QT Interval." Am J Med Sci 327 (2004): 55-6. [PMID: 14722399] | ||||
| 92 | Bluhm RE, Wilkinson GR, Shelton R, Branch RA "Genetically determined drug-metabolizing activity and desipramine- associated cardiotoxicity: a case report." Clin Pharmacol Ther 53 (1993): 89-95. [PMID: 8422746] | ||||
| 93 | Product Information. Barhemsys (amisulpride). Acacia Pharma, Inc, Indianapolis, IN. | ||||
| 94 | Goto M, Sato M, Kitzazawa H, et.al "Papaverine-induced QT interval prolongation and ventricular fibrillation in a patient with a history of drug-induced QT prolongation." Intern Med 53 (2014): 1629-31. [PMID: 25088875] | ||||
| 95 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
| 96 | Lee BS "Possibility of hyperpyrexia with antipsychotic and anticholinergic drugs." J Clin Psychiatry 47 (1986): 571. [PMID: 3771507] | ||||
| 97 | Katz MR "Raised serum levels of desipramine with the antiarrhythmic propafenone ." J Clin Psychiatry 52 (1991): 432-3. [PMID: 1938981] | ||||
