Details of the Drug
General Information of Drug (ID: DMYZ57N)
| Drug Name |
Albendazole
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Albendazol; Albendazolum; Albendoral; Albenza; Bendapar; Bilutac; Digezanol; Disthelm; Endoplus; Eskazole; Gascop; Lurdex; Metiazol; Proftril; Valbazen; Zental; Zentel; Albendazole Armstrong Brand; Albendazole Diba Brand; Albendazole Fustery Brand; Albendazole Hormona Brand; Albendazole Liferpal Brand; Albendazole Monohydrochloride; Albendazole Pfizer Brand; Albendazole Sanicoopa Brand; Albendazole Valdecasas Brand; Armstrong Brand of Albendazole; Diba Brand of Albendazole; Fustery Brand of Albendazole; Hormona Brand of Albendazole; Liferpal Brand of Albendazole; Mediamix V Disthelm; Noe Socopharm Brand of Albendazole; Pfizer Brand of Albendazole; Sanicoopa Brand of Albendazole; SmithKline Beecham Brand of Albendazole; Valdecasas Brand of Albendazole; SKF 62979; SKF62979; Albendazol [INN-Spanish]; Albendazole Noe-Socopharm Brand; Albendazolum [INN-Latin]; Albenza (TN); Disthelm, Mediamix V; Eskazole (TN); Monohydrochloride, Albendazole; Noe-Socopharm Brand of Albendazole; SK&F 62979; SK&F62979; SKF-62979; Smith Kline & French Brand of Albendazole; V Disthelm, Mediamix; Zentel (TN); SK&F-62979; Albendazole (JAN/USP/INN); Albendazole [USAN:INN:BAN:JAN]; Albenza, Eskazole, Zentel, Andazol, Albendazole; Methyl 5-(propylthio)-2-benzimidazolecarbamate; Methyl [5-(Propylthio)benzimidazol-2-yl]carbamate; Methyl-5-[propylthio]-2-benzimidazole carbamate; [5-(Propylthio)benzimidazol-2-yl]carbamic Acid Methyl Ester; Methyl 5-(propyl-thio)-2-benzimidazolecarbamate; Methyl [5-(propylsulfanyl)-1H-benzimidazol-2-yl]carbamate; Methyl [5-(propylthio)-1H-benzimidazol-2-yl]carbamate; Methyl [6-(propylsulfanyl)-1H-benzimidazol-2-yl]carbamate; [5-(Propythio)-1H-benzimidazol-2-yl]carbamic acid methyl ester; MethylN-(6-propylsulfanyl-1H-benzimidazol-2-yl)carbamate; O-Methyl N-(5-(propylthio)-2-benzimidazolyl)carbamate; Carbamic acid, [5-(propylthio)-1H-benzimidazol-2-yl]-, methyl ester; Methyl N-[6-(propylsulfanyl)-1H-1,3-benzodiazol-2-yl]carbamate; CARBAMIC ACID, (5-(PROPYLTHIO)-1H-BENZIMIDAZOL-2-YL)-, METHYL ESTER; Methyl [(2Z)-5-(propylsulfanyl)-1,3-dihydro-2H-benzimidazol-2-ylidene]carbamate; ((Propylthio)-5 1H-benzimidazolyl-2) carbamate de methyle; ((Propylthio)-5 1H-benzimidazolyl-2) carbamate de methyle [French]; (5-(Propylthio)-1H-benzimidazol-2-yl)carbamic acid methyl ester; (5-Propylsulfanyl-1H-benzoimidazol-2-yl)-carbamic acid methyl ester; 5-(Propylthio)-2-carbomethoxyaminobenzimidazole
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiprotozoal Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Helminthic Microorganisms
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
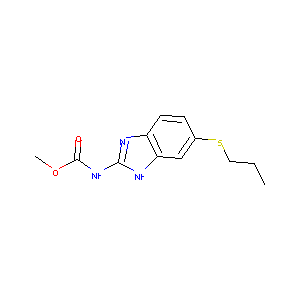 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 265.33 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.9 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Albendazole (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Albendazole FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discov. 2005 Sep;4(9):727-40. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Flubendazole interferes with a wide spectrum of cell homeostatic mechanisms in Echinococcus granulosus protoscoleces. Parasitol Int. 2009 Sep;58(3):270-7. | ||||
| 7 | The anthelminthic agent albendazole does not interact with p-glycoprotein. Drug Metab Dispos. 2002 Apr;30(4):365-9. | ||||
| 8 | Identification of human cytochrome P(450)s that metabolise anti-parasitic drugs and predictions of in vivo drug hepatic clearance from in vitro data. Eur J Clin Pharmacol. 2003 Sep;59(5-6):429-42. | ||||
| 9 | Danazol inhibits cytochrome P450 2J2 activity in a substrate-independent manner. Drug Metab Dispos. 2015 Aug;43(8):1250-3. | ||||
| 10 | Cytochrome P450 1A1/2 induction by antiparasitic drugs: dose-dependent increase in ethoxyresorufin O-deethylase activity and mRNA caused by quinine, primaquine and albendazole in HepG2 cells. Eur J Clin Pharmacol. 2002 Nov;58(8):537-42. | ||||
| 11 | Cell-based and cytokine-directed chemical screen to identify potential anti-multiple myeloma agents. Leuk Res. 2010 Jul;34(7):917-24. doi: 10.1016/j.leukres.2009.12.002. Epub 2010 Feb 8. | ||||
| 12 | Identification of environmental chemicals that activate p53 signaling after in vitro metabolic activation. Arch Toxicol. 2022 Jul;96(7):1975-1987. doi: 10.1007/s00204-022-03291-5. Epub 2022 Apr 18. | ||||
| 13 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 14 | A Gene Expression Biomarker Predicts Heat Shock Factor 1 Activation in a Gene Expression Compendium. Chem Res Toxicol. 2021 Jul 19;34(7):1721-1737. doi: 10.1021/acs.chemrestox.0c00510. Epub 2021 Jun 25. | ||||
| 15 | Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3. | ||||
| 16 | Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression. Mol Neurodegener. 2006 Jul 26;1:6. doi: 10.1186/1750-1326-1-6. | ||||
| 17 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 18 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 19 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 20 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 21 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 22 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 23 | Product Information. Chirocaine (levobupivacaine) Organon, West Orange, NJ. | ||||
| 24 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 25 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 26 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 27 | Product Information. Trileptal (oxcarbazepine) Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 28 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 29 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 30 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 31 | Auclair B, Berning SE, Huitt GA, Peloquin CP "Potential interaction between itraconazole and clarithromycin." Pharmacotherapy 19 (1999): 1439-44. [PMID: 10600094] | ||||
| 32 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 33 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 34 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 35 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 36 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 37 | Product Information. Sustiva (efavirenz). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 38 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 39 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 40 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 41 | Product Information. Hetlioz (tasimelteon). Vanda Pharmaceuticals Inc, Rockville, MD. | ||||
| 42 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 43 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 44 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 45 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 46 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 47 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 48 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 49 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 50 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 51 | Product Information. Rozlytrek (entrectinib). Genentech, South San Francisco, CA. | ||||
| 52 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 53 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 54 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 55 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 56 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 57 | Product Information. Tracleer (bosentan). Acetelion Pharmaceuticals US, Inc, South San Francisco, CA. | ||||
| 58 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 59 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 60 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 61 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
