| 1 |
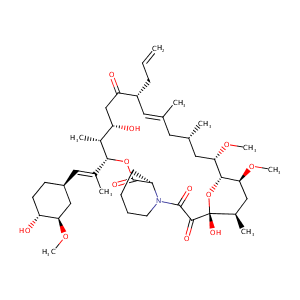
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Tacrolimus FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6784).
|
| 4 |
The fight against drug-resistant malaria: novel plasmodial targets and antimalarial drugs. Curr Med Chem. 2008;15(2):161-71.
|
| 5 |
GSK3, snail, and adhesion molecule regulation by cyclosporine A in renal tubular cells. Toxicol Sci. 2012 Jun;127(2):425-37. doi: 10.1093/toxsci/kfs108. Epub 2012 Mar 12.
|
| 6 |
Calcineurin is an important factor involved in glucose uptake in human adipocytes. Mol Cell Biochem. 2018 Aug;445(1-2):157-168. doi: 10.1007/s11010-017-3261-0. Epub 2018 Jan 27.
|
| 7 |
Emerging drugs for ocular allergy. Expert Opin Emerg Drugs. 2005 Aug;10(3):505-20.
|
| 8 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 9 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 10 |
Tacrolimus pharmacokinetics and pharmacogenetics: influence of adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome (CYP) 3A polymorphisms. Fundam Clin Pharmacol. 2007 Aug;21(4):427-35.
|
| 11 |
Polymorphisms in cytochrome P450 oxidoreductase and its effect on drug metabolism and efficacy. Pharmacogenet Genomics. 2017 Sep;27(9):337-346.
|
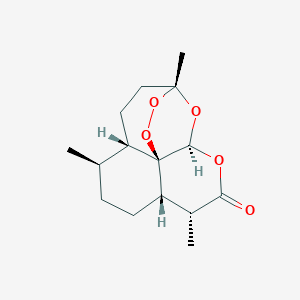
| 12 |
High frequency of macrolide resistance mechanisms in clinical isolates of Corynebacterium species. Microb Drug Resist. 2010 Dec;16(4):273-7.
|
| 13 |
Contribution of CYP3A5 to the in vitro hepatic clearance of tacrolimus. Clin Chem. 2005 Aug;51(8):1374-81.
|
| 14 |
Single-nucleotide polymorphisms in P450 oxidoreductase and peroxisome proliferator-activated receptor- are associated with the development of new-onset diabetes after transplantation in kidney transplant recipients treated with tacrolimus. Pharmacogenet Genomics. 2013 Dec;23(12):649-57. doi: 10.1097/FPC.0000000000000001.
|
| 15 |
Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation. 2002 Aug 27;74(4):571-2. doi: 10.1097/00007890-200208270-00024.
|
| 16 |
Oxidative stress in kidney transplant patients with calcineurin inhibitor-induced hypertension: effect of ramipril. J Cardiovasc Pharmacol. 2002 Oct;40(4):625-31.
|
| 17 |
A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci. 2013 Nov;136(1):216-41.
|
| 18 |
Mycophenolic acid inhibits natural killer cell proliferation and cytotoxic function: a possible disadvantage of including mycophenolate mofetil in the graft-versus-host disease prophylaxis regimen. Biol Blood Marrow Transplant. 2011 Feb;17(2):205-13. doi: 10.1016/j.bbmt.2010.08.014. Epub 2010 Aug 22.
|
| 19 |
CyclinB2 and BIRC5 genes as surrogate biomarkers for neurite outgrowth in SH-SY5Y subclonal cells. Neuropharmacology. 2006 Jun;50(8):1041-7. doi: 10.1016/j.neuropharm.2006.02.004. Epub 2006 Mar 30.
|
| 20 |
Effects of immunosuppressive drugs on in vitro neogenesis of human islets: mycophenolate mofetil inhibits the proliferation of ductal cells. Am J Transplant. 2007 Apr;7(4):1021-6. doi: 10.1111/j.1600-6143.2006.01728.x.
|
| 21 |
FK506 inhibits tumour necrosis factor-alpha secretion in human keratinocytes via regulation of nuclear factor-kappaB. Br J Dermatol. 2005 Oct;153(4):725-32. doi: 10.1111/j.1365-2133.2005.06779.x.
|
| 22 |
Tacrolimus suppressed the production of cytokines involved in atopic dermatitis by direct stimulation of human PBMC system. (Comparison with steroids). Int Immunopharmacol. 2001 Jun;1(6):1219-26. doi: 10.1016/s1567-5769(01)00059-5.
|
| 23 |
Profiling the immunotoxicity of chemicals based on in vitro evaluation by a combination of the Multi-ImmunoTox assay and the IL-8 Luc assay. Arch Toxicol. 2018 Jun;92(6):2043-2054. doi: 10.1007/s00204-018-2199-7. Epub 2018 Mar 29.
|
| 24 |
Tacrolimus-induced nephrotoxicity in mice is associated with microRNA deregulation. Arch Toxicol. 2018 Apr;92(4):1539-1550. doi: 10.1007/s00204-018-2158-3. Epub 2018 Jan 23.
|
| 25 |
Tacrolimus decreases the expression of eotaxin, CCR3, RANTES and interleukin-5 in atopic dermatitis. Br J Dermatol. 2005 Jun;152(6):1173-81. doi: 10.1111/j.1365-2133.2005.06474.x.
|
| 26 |
A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro. J Am Soc Nephrol. 2016 Apr;27(4):1015-28. doi: 10.1681/ASN.2015010060. Epub 2015 Aug 10.
|
| 27 |
Tacrolimus ointment causes inflammatory dendritic epidermal cell depletion but no Langerhans cell apoptosis in patients with atopic dermatitis. J Allergy Clin Immunol. 2004 Jul;114(1):137-43. doi: 10.1016/j.jaci.2004.03.021.
|
| 28 |
Immunosuppressive calcineurin inhibitor cyclosporine A?induces proapoptotic endoplasmic reticulum stress in renal tubular cells. J Biol Chem. 2022 Mar;298(3):101589. doi: 10.1016/j.jbc.2022.101589. Epub 2022 Jan 14.
|
| 29 |
Multicenter prospective investigation on cardiovascular adverse effects of tacrolimus in kidney transplantations. Cardiovasc Drugs Ther. 2003 Mar;17(2):141-9. doi: 10.1023/a:1025339819051.
|
| 30 |
Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol. 2001 Nov;108(5):839-46. doi: 10.1067/mai.2001.118796.
|
| 31 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 32 |
Hydrogen peroxide mediates FK506-induced cytotoxicity in renal cells. Kidney Int. 2004 Jan;65(1):139-47. doi: 10.1111/j.1523-1755.2004.00380.x.
|
| 33 |
Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2005 Jun;67(6):1954-65.
|
| 34 |
Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br J Clin Pharmacol. 1999 Oct;48(4):528-35.
|
| 35 |
Inhibition of glutathione S-transferases by antimalarial drugs possible implications for circumventing anticancer drug resistance. Int J Cancer. 2002 Feb 10;97(5):700-5.
|
| 36 |
Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2005 Jun;67(6):1954-65.
|
| 37 |
Application of higher throughput screening (HTS) inhibition assays to evaluate the interaction of antiparasitic drugs with cytochrome P450s. Drug Metab Dispos. 2001 Jan;29(1):30-5.
|
| 38 |
Identification of Compounds That Inhibit Estrogen-Related Receptor Alpha Signaling Using High-Throughput Screening Assays. Molecules. 2019 Feb 27;24(5):841. doi: 10.3390/molecules24050841.
|
| 39 |
In vivo and mechanistic evidence of nuclear receptor CAR induction by artemisinin. Eur J Clin Invest. 2006 Sep;36(9):647-53. doi: 10.1111/j.1365-2362.2006.01700.x.
|
|
|
|
|
|
|