Details of the Drug
General Information of Drug (ID: DM3UZ95)
| Drug Name |
Bisoprolol
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bisocor; Bisoprololum; Cardicor; Concor; Detensiel; Emconcor; Emcor; Euradal; Isoten; Monocor; Soloc; Soprol; Zebeta; Bisoprolol fumerate; Bisoprolol hemifumarate; Bisoprololum [Latin]; CL-297939; Concor (TN); Concore (TN); EMD-33512; Monocor (TN); Zebeta (TN); Bisoprolol (USAN/INN); Bisoprolol [USAN:BAN:INN]; EMD-33-512; (+-)-1-((alpha-(2-Isopropoxyethoxy)-p-tolyl)oxy)-3-(isopropylamino)-2-propanol; (RS)-1-(4-(2-Isopropoxyethoxymethyl)phenoxy)-3-(isopropylamino)-2-propanol; 1-(propan-2-ylamino)-3-[4-(2-propan-2-yloxyethoxymethyl)phenoxy]propan-2-ol; 1-[(1-methylethyl)amino]-3-({4-[({2-[(1-methylethyl)oxy]ethyl}oxy)methyl]phenyl}oxy)propan-2-ol; 1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3-(isopropylamino)propan-2-ol
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
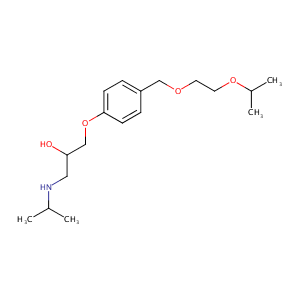 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 325.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 12 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Bisoprolol
Coadministration of a Drug Treating the Disease Different from Bisoprolol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7129). | ||||
|---|---|---|---|---|---|
| 2 | Bisoprolol FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Kirch W, Rose I, Demers HG, Leopold G, Pabst J, Ohnhaus EE: Pharmacokinetics of bisoprolol during repeated oral administration to healthy volunteers and patients with kidney or liver disease. Clin Pharmacokinet. 1987 Aug;13(2):110-7. doi: 10.2165/00003088-198713020-00003. | ||||
| 5 | Bisoprolol monograph | ||||
| 6 | Pharmacodynamic profile of bisoprolol, a new beta 1-selective adrenoceptor antagonist. Br J Clin Pharmacol. 1986 Sep;22(3):293-300. doi: 10.1111/j.1365-2125.1986.tb02890.x. | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 9 | Summary of product characteristics | ||||
| 10 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 11 | Antiarrhythmic effect of bisoprolol, a highly selective beta1-blocker, in patients with paroxysmal atrial fibrillation. Int Heart J. 2008 May;49(3):281-93. | ||||
| 12 | Stereoselective metabolism of bisoprolol enantiomers in dogs and humans. Life Sci. 1998;63(13):1097-108. | ||||
| 13 | Drug-drug interactions of beta-adrenoceptor blockers. Arzneimittelforschung. 2003;53(12):814-22. | ||||
| 14 | Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam Clin Pharmacol. 2006 Jun;20(3):273-82. doi: 10.1111/j.1472-8206.2006.00408.x. | ||||
| 15 | The effects of 1 month antihypertensive treatment with perindopril, bisoprolol or both on the ex vivo ability of monocytes to secrete inflammatory cytokines. Int J Clin Pharmacol Ther. 2009 Nov;47(11):686-94. doi: 10.5414/cpp47686. | ||||
| 16 | Anastassiades CJ "Nifedipine and beta-blocker drugs." Br Med J 281 (1980): 1251-2. [PMID: 6107167] | ||||
| 17 | Dean S, Kendall MJ, Potter S, Thompson MH, Jackson DA "Nadolol in combination with indapamide and xipamide in resistant hypertensives." Eur J Clin Pharmacol 28 (1985): 29-33. [PMID: 3987783] | ||||
| 18 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 19 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 20 | Product Information. Zylo Filmtab (zileuton). Abbott Pharmaceutical, Abbott Park, IL. | ||||
| 21 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 22 | Chapple DJ, Clark JS, Hughes R "Interaction between atracurium and drugs used in anaesthesia." Br J Anaesth 55 Suppl 1 (1983): s17-22. [PMID: 6688011] | ||||
| 23 | Canadian Pharmacists Association. | ||||
| 24 | Chrysant SG "Experience with terazosin administered in combination with other antihypertensive agents." Am J Med 80 (1986): 55-61. [PMID: 2872808] | ||||
| 25 | Kirch W, Rose I, Klingmann I, Pabst J, Ohnhaus EE "Interaction of bisoprolol with cimetidine and rifampicin." Eur J Clin Pharmacol 31 (1986): 59-62. [PMID: 2877885] | ||||
| 26 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 27 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 28 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 29 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 30 | Leor J, Levartowsky D, Sharon C, Farfel Z "Amiodarone and beta-adrenergic blockers: an interaction with metoprolol but not with atenolol." Am Heart J 16 (1988): 206-7. [PMID: 3394625] | ||||
