| 1 |
Biologically active neutrophil chemokine pattern in tonsillitis.Clin Exp Immunol. 2004 Mar;135(3):511-8. doi: 10.1111/j.1365-2249.2003.02390.x.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5692).
|
| 3 |
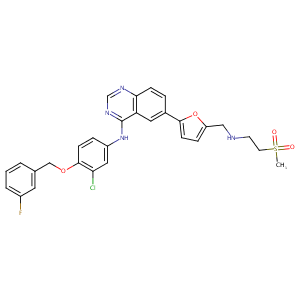
Lapatinib FDA Label
|
| 4 |
Pomalidomide FDA Label
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7348).
|
| 6 |
UGT-dependent regioselective glucuronidation of ursodeoxycholic acid and obeticholic acid and selective transport of the consequent acyl glucuronides by OATP1B1 and 1B3. Chem Biol Interact. 2019 Sep 1;310:108745. doi: 10.1016/j.cbi.2019.108745. Epub 2019 Jul 9.
|
| 7 |
Triple negative breast cancer--current status and prospective targeted treatment based on HER1 (EGFR), TOP2A and C-MYC gene assessment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009 Mar;153(1):13-7.
|
| 8 |
Inhibition of eEF-2 kinase sensitizes human nasopharyngeal carcinoma cells to lapatinib-induced apoptosis through the Src and Erk pathways.BMC Cancer. 2016 Oct 19;16(1):813.
|
| 9 |
Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition.
|
| 10 |
The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008 Apr;36(4):695-701.
|
| 11 |
Mechanism-based inactivation of cytochrome P450 3A4 by lapatinib. Mol Pharmacol. 2010 Oct;78(4):693-703.
|
| 12 |
Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009 Dec;35(8):692-706.
|
| 13 |
The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005 Jan 1;65(1):18-25.
|
| 14 |
Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500.
|
| 15 |
P450 3A-catalyzed O-dealkylation of lapatinib induces mitochondrial stress and activates Nrf2. Chem Res Toxicol. 2016 May 16;29(5):784-96.
|
| 16 |
Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005 Sep 15;24(41):6213-21. doi: 10.1038/sj.onc.1208774.
|
| 17 |
CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer. 2014 Jul;5(7-8):261-72. doi: 10.18632/genesandcancer.24.
|
| 18 |
Effects of lapatinib on cell proliferation and apoptosis in NB4 cells. Oncol Lett. 2018 Jan;15(1):235-242. doi: 10.3892/ol.2017.7342. Epub 2017 Nov 3.
|
| 19 |
The involvement of hepatic cytochrome P450s in the cytotoxicity of lapatinib. Toxicol Sci. 2023 Dec 21;197(1):69-78. doi: 10.1093/toxsci/kfad099.
|
| 20 |
Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4.
|
| 21 |
Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009 Jun 15;15(12):4147-56. doi: 10.1158/1078-0432.CCR-08-2814. Epub 2009 Jun 9.
|
| 22 |
Cytotoxicity of 34 FDA approved small-molecule kinase inhibitors in primary rat and human hepatocytes. Toxicol Lett. 2018 Jul;291:138-148. doi: 10.1016/j.toxlet.2018.04.010. Epub 2018 Apr 12.
|
| 23 |
Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167.
|
| 24 |
The K-Ras effector p38 MAPK confers intrinsic resistance to tyrosine kinase inhibitors by stimulating EGFR transcription and EGFR dephosphorylation. J Biol Chem. 2017 Sep 8;292(36):15070-15079. doi: 10.1074/jbc.M117.779488. Epub 2017 Jul 24.
|
| 25 |
HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J Clin Oncol. 2011 Feb 20;29(6):667-73. doi: 10.1200/JCO.2010.31.3197. Epub 2011 Jan 18.
|
| 26 |
Niclosamide inhibits epithelial-mesenchymal transition and tumor growth in lapatinib-resistant human epidermal growth factor receptor 2-positive breast cancer. Int J Biochem Cell Biol. 2016 Feb;71:12-23. doi: 10.1016/j.biocel.2015.11.014. Epub 2015 Nov 28.
|
| 27 |
Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651.
|
| 28 |
DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB08910)
|
| 29 |
Pomalidomide: evaluation of cytochrome P450 and transporter-mediated drug-drug interaction potential in vitro and in healthy subjects. J Clin Pharmacol. 2015 Feb;55(2):168-78.
|
| 30 |
Population pharmacokinetics of pomalidomide. J Clin Pharmacol. 2015 May;55(5):563-72.
|
| 31 |
Human cytochrome P450 oxidation of 5-hydroxythalidomide and pomalidomide, an amino analogue of thalidomide. Chem Res Toxicol. 2014 Jan 21;27(1):147-56. doi: 10.1021/tx4004215. Epub 2013 Dec 24.
|
| 32 |
Immunomodulatory derivative of thalidomide (IMiD CC-4047) induces a shift in lineage commitment by suppressing erythropoiesis and promoting myelopoiesis. Blood. 2005 May 15;105(10):3833-40. doi: 10.1182/blood-2004-03-0828. Epub 2004 Aug 3.
|
| 33 |
Thalidomide and Its Analogs Differentially Target Fibroblast Growth Factor Receptors: Thalidomide Suppresses FGFR Gene Expression while Pomalidomide Dampens FGFR2 Activity. Chem Res Toxicol. 2019 Apr 15;32(4):589-602. doi: 10.1021/acs.chemrestox.8b00286. Epub 2019 Mar 15.
|
| 34 |
Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. Elife. 2018 Aug 1;7:e38430. doi: 10.7554/eLife.38430.
|
| 35 |
A Dual Color Immunohistochemistry Assay for Measurement of Cereblon in Multiple Myeloma Patient Samples. Appl Immunohistochem Mol Morphol. 2016 Nov/Dec;24(10):695-702. doi: 10.1097/PAI.0000000000000246.
|
| 36 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
|
|
|
|
|
|