Details of the Drug
General Information of Drug (ID: DMKJ485)
| Drug Name |
Pioglitazone
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
111025-46-8; Actos; Pioglitazona; Pioglitazonum; Glustin; Zactos; 105355-27-9; Pioglitazonum [INN-Latin]; Pioglitazona [INN-Spanish]; Duetact; Pioglitazone [INN:BAN]; Pioglitazone [BAN:INN]; 5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzyl)thiazolidine-2,4-dione; AD-4833; U 72107; CHEBI:8228; Pioglitazone (Actos); HSDB 7322; Actos (TN); 5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione; C19H20N2O3S; AD 4833; 5-[4-[2-(5-ETHYL-2-PYRIDYL)ETHOXY]BENZYL]-2,4-THIAZOLIDINEDIONE; U 72107A; Actos; Actost; Glustin (TN); HS-0047; Pioglitazone (INN); U-72107; U72,107A; Zactos (TN); (+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione; (+/-)-5-[[4-[2-(5-Ethyl-2-pyridinyl)-ethoxy]phenyl]methyl]-2,4-thiazolidinedione; (+/-)-5-[p-[2-(ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione; 2,4-Thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-(9CI); 5-((4-(2-(5-Ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione; 5-(4-(2-(5-ethyl-2-pyridyl)ethoxy)benzyl)-2,4-thiazolidinedione; 5-[4-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl]thiazolidine-2,4-dione; 5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]thiazolidine-2,4-dione; 5-[[4-[2-[(5-ethyl-2-pyridyl)]ethoxy]phenyl]methyl]thiazolidine-2,4-dione; Linagliptin + pioglitazone; PCG1
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Hypoglycemic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
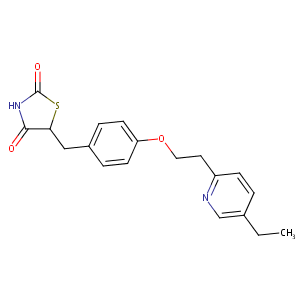 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 356.4 | ||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | |||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | |||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 16 Disease of the genitourinary system | |||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: GA10 Endometriosis | |||||||||||||||||||||||||||||||||||||||||||||||
| The Studied Tissue | Endometrium tissue | |||||||||||||||||||||||||||||||||||||||||||||||
| The Studied Disease | Endometriosis [ICD-11:GA10] | |||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Pioglitazone (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Pioglitazone FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2694). | ||||
| 3 | ClinicalTrials.gov (NCT01183013) 30 Week Parallel Group Comparison Study of Linagliptin + Pioglitazone (5+15, 5+30 and 5+45 mg) qd Versus Respective Monotherapies, Followed by a Comparison of 5mg+30mg and 5mg+45mg Versus Respective Monotherapies in Type 2 Diabetes for up to 54 Weeks. U.S. National Institutes of Health. | ||||
| 4 | Obesity: pathophysiology and clinical management. Curr Med Chem. 2009;16(4):506-21. | ||||
| 5 | FDA Approved Drug Products: Actos (pioglitazone) oral tablets | ||||
| 6 | BDDCS applied to over 900 drugs | ||||
| 7 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 8 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 9 | Functional PPAR-gamma receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med. 2002 Nov;8(11):1281-7. | ||||
| 10 | Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol. 2006 Jul;99(1):44-51. | ||||
| 11 | Current clinical evidence on pioglitazone pharmacogenomics. Front Pharmacol. 2013 Nov 26;4:147. | ||||
| 12 | The role of human CYP2C8 and CYP2C9 variants in pioglitazone metabolism in vitro. Basic Clin Pharmacol Toxicol. 2009 Dec;105(6):374-9. | ||||
| 13 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 14 | Pioglitazone inhibits androgen production in NCI-H295R cells by regulating gene expression of CYP17 and HSD3B2. Mol Pharmacol. 2007 Mar;71(3):787-98. | ||||
| 15 | PPAR gamma ligands, troglitazone and pioglitazone, up-regulate expression of HMG-CoA synthase and HMG-CoA reductase gene in THP-1 macrophages. FEBS Lett. 2002 Jun 5;520(1-3):177-81. | ||||
| 16 | Peroxisome proliferator activated receptor gamma (PPAR-gama) ligand pioglitazone regulated gene networks in term human primary trophoblast cells. Reprod Toxicol. 2018 Oct;81:99-107. | ||||
| 17 | Comparison of the effects of pioglitazone and rosiglitazone on macrophage foam cell formation. Biochem Biophys Res Commun. 2004 Oct 22;323(3):782-8. | ||||
| 18 | PARP-1 suppresses adiponectin expression through poly(ADP-ribosyl)ation of PPAR gamma in cardiac fibroblasts. Cardiovasc Res. 2009 Jan 1;81(1):98-107. doi: 10.1093/cvr/cvn264. Epub 2008 Sep 24. | ||||
| 19 | Co-Administration of Melatonin Effectively Enhances the Therapeutic Effects of Pioglitazone on Mesenchymal Stem Cells Undergoing Indoxyl Sulfate-Induced Senescence through Modulation of Cellular Prion Protein Expression. Int J Mol Sci. 2018 May 4;19(5):1367. doi: 10.3390/ijms19051367. | ||||
| 20 | Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005 Jun;128(Pt 6):1442-53. doi: 10.1093/brain/awh452. Epub 2005 Apr 7. | ||||
| 21 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 22 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 23 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 24 | Product Information. Actos (pioglitazone) Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 25 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 26 | Aquilante CL, Kosmiski LA, Bourne DW, et al. "Impact of the CYP2C8 *3 polymorphism on the drug-drug interaction between gemfibrozil and pioglitazone." Br J Clin Pharmacol 75 (2013): 217-26. [PMID: 22625877] | ||||
| 27 | Glazer NB, Cheatham WW "Thiazolidinediones for type 2 diabetes - No evidence exists that pioglitazone induces hepatic cytochrome P450 isoform CYP3A4." Br Med J 322 (2001): 235-6. [PMID: 11159615] | ||||
| 28 | Loi CM, Stern R, Koup JR, Vassos AB, Knowlton P, Sedman AJ "Effect of troglitazone on the pharmacokinetics of an oral contraceptive agent." J Clin Pharmacol 39 (1999): 410-7. [PMID: 10197300] | ||||
| 29 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 30 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 31 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 32 | Jaakkola T, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ "Effect of rifampicin on the pharmacokinetics of pioglitazone." Br J Clin Pharmacol 61 (2006): 70-8. [PMID: 16390353] | ||||
| 33 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 34 | Product Information. Intelence (etravirine). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 35 | Canadian Pharmacists Association. | ||||
| 36 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 37 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 38 | Product Information. Caplyta (lumateperone). Intra-Cellular Therapies, Inc., New York, NY. | ||||
| 39 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 40 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 41 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 42 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 43 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 44 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 45 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
