Details of the Drug
General Information of Drug (ID: DMSOX7I)
| Drug Name |
Cocaine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Allococaine; Allopseudococaine; Badrock; Bazooka; Benzoylethylecgonine; Benzoylmethylecgonine; Bernice; Bernies; Blast; Blizzard; Blow; Burese; COC; Cabello; Candy; Carrie; Caviar; Cecil; Charlie; Cholly; Coca; Cocain; Cocaina; Cocainum; Cocktail; Coke; Cola; Corine; Crack; Eritroxilina; Erytroxylin; Flake; Flex; Freeze; Girl; Goofball; Heaven; Hell; Jam; Kokain; Kokan; Kokayeen; Lady; Leaf; Moonrocks; Neurocaine; Pseudoallococaine; Rock; Sleighride; Snort; Snow; Toke; Toot; Trails; Yeyo; Bernice [Street Name]; Blow [Street Name]; Bouncing Powder; Cecil [Street Name]; Chicken Scratch; Cocaine [BAN]; Cocaine free base; Cocaine solution; Crack cocaine; Dama blanca; Ecgonine methyl ester benzoate; Ecgonine methyl ester benzoate solution; Flake [Street Name]; Florida Snow; Foo Foo; Girl [Street Name]; Gold dust; Gold dust [Street Name]; Green gold; Happy dust; Happy dust [Street Name]; Happy powder; Happy trails; Lady [Street Name]; Methyl Benzoylecgonine; Nose candy; Prime Time; Rock [Street Name]; Sweet Stuff; Toot [Street Name]; White girl or lady; Beta-Cocain; C" Carrie; Cocaine (TN); Cocaine (USP); Cocaine-M; G-Rock; Kibbles n' Bits; L-Cocain; L-Cocaine; Pimp's drug; Snow (birds); Star-spangled powder; Ecgonine, methyl ester, benzoate (ester); Methyl 3beta-hydroxy-1alphaH,5alphaH-tropane-2beta-carboxylate benzoate (ester); Methyl 3-benzoyloxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate; Methyl 3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate; Methyl (3S,4R)-3-benzoyloxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate; Methyl (1S,4R,5R)-3-benzoyloxy-8-methyl-8-azabicyclo[3.2.1]octane-4-carboxylate; (-)-Cocaine; (-)-Cocaine base; (1R,2R,3S,5S)-2-(methoxycarbonyl)tropan-3-yl benzoate; (1R,2R,3S,5S)-2-Methoxycarbonyltropan-3-yl benzoate; (R)-Cocaine; 1-Cocaine; 2-beta-Carbomethoxy-3-beta-benzoxytropane; 2-beta-Tropanecarboxylic acid, 3-beta-hydroxy-, methyl ester, benzoate (ester); 2beta-Carbomethoxy-3beta-benzoxytropane; 3-Tropanylbenzoate-2-carboxylic acid methyl ester; 3beta-Hydroxy-2beta-tropanecarboxylic acid methyl ester benzoate (ester)
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
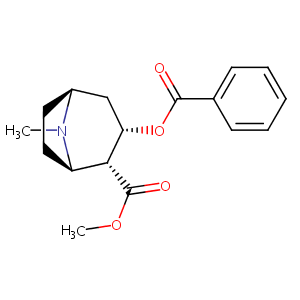 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 303.35 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Anaesthesia | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 9A78.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Cocaine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
