Details of the Drug
General Information of Drug (ID: DM05FXR)
| Drug Name |
Meclofenamic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Arquel; Meclofenamate; Acide meclofenamique; Acido meclofenamico; Acidum meclofenamicum; Meclophenamic acid; CL 583; INF 4668; Acide meclofenamique [INN-French]; Acido meclofenamico [INN-Spanish]; Acidum meclofenamicum [INN-Latin]; INF-4668; Meclomen (free acid); Meclofenamic acid (USAN/INN); Meclofenamic acid [USAN:INN:BAN]; N-(2,6-Dichloro-3-methylphenyl)anthranilic acid; N-(2,6-Dichloro-m-tolyl)anthranilic acid; N-(3-Methyl-2,6-dichlorophenyl)anthranilic acid; 2-((2,6-Dichloro-3-methylphenyl)amino)benzoic acid; 2-(2,6-Dichloro-3-methylphenyl)aminobenzoic acid; 2-(2,6-dichloro-3-methylanilino)benzoic acid; 2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
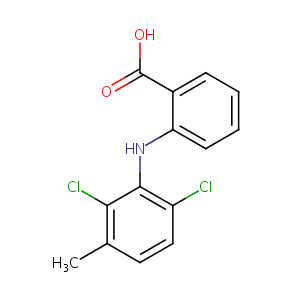 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 296.1 | ||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Meclofenamic acid (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7219). | ||||
|---|---|---|---|---|---|
| 2 | Use of meclofenamic acid in gynecology and obstetrics: effects on postsurgical stress. Clin J Pain. 1991;7 Suppl 1:S60-3. | ||||
| 3 | Quantitation of indomethacin in serum and plasma using gas chromatography-mass spectrometry (GC-MS). Methods Mol Biol. 2010;603:297-305. | ||||
| 4 | Meclofenamic Acid Restores Gefinitib Sensitivity by Downregulating Breast Cancer Resistance Protein and Multidrug Resistance Protein 7 via FTO/m6A-Demethylation/c-Myc in Non-Small Cell Lung Cancer. Front Oncol. 2022 Apr 21;12:870636. | ||||
| 5 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 6 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 7 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 8 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 9 | Interactions of PGH synthase isozymes-1 and -2 with NSAIDs. Ann N Y Acad Sci. 1994 Nov 15;744:50-7. | ||||
| 10 | Both reactivity and accessibility are important in cytochrome P450 metabolism: a combined DFT and MD study of fenamic acids in BM3 mutants. J Chem Inf Model. 2019 Feb 25;59(2):743-753. | ||||
| 11 | Type 5 17beta-hydroxysteroid dehydrogenase/prostaglandin F synthase (AKR1C3): role in breast cancer and inhibition by non-steroidal anti-inflammatory drug analogs. Chem Biol Interact. 2009 Mar 16;178(1-3):221-7. | ||||
| 12 | Initro CAPE inhibitory activity towards human AKR1C3 and the molecular basis. Chem Biol Interact. 2016 Jun 25;253:60-5. | ||||
| 13 | Structure-function relationship and role of tumor necrosis factor-alpha-converting enzyme in the down-regulation of L-selectin by non-steroidal anti-inflammatory drugs. J Biol Chem. 2002 Oct 11;277(41):38212-21. doi: 10.1074/jbc.M205142200. Epub 2002 Jul 29. | ||||
| 14 | Meclofenamate sodium is an inhibitor of both the 5-lipoxygenase and cyclooxygenase pathways of the arachidonic acid cascade in vitro. Prostaglandins Leukot Med. 1986 Aug;23(2-3):229-38. | ||||
| 15 | Inhibition of human phenol and estrogen sulfotransferase by certain non-steroidal anti-inflammatory agents. Curr Drug Metab. 2006 Oct;7(7):745-53. | ||||
| 16 | European Medicines Agency "Summary on compassionate use. Remdesivir Gilead.". | ||||
| 17 | Christensen LK, Hansen JM, Kristensen M "Sulphaphenazole-induced hypoglycemic attacks in tolbutamide-treated diabetics." Lancet 2 (1963): 1298-301. [PMID: 14071924] | ||||
| 18 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 19 | Product Information. Hivid (zalcitabine). Roche Laboratories, Nutley, NJ. | ||||
| 20 | Product Information. Acular (ketorolac). Allergan Inc, Irvine, CA. | ||||
| 21 | Messer J, Reitman D, Sacks HS, et al "Association of adrenocorticosteroid therapy and peptic-ulcer disease." N Engl J Med 309 (1983): 21-4. [PMID: 6343871] | ||||
| 22 | Davey PG "Overview of drug interactions with the quinolones." J Antimicrob Chemother 22(suppl c) (1988): 97-107. [PMID: 3053579] | ||||
| 23 | Farag MM, Mikhail MR, Abdel-Meguid E, Abdel-Tawab S "Assessment of gentamicin-induced nephrotoxicity in rats treated with low doses of ibuprofen and diclofenac sodium." Clin Sci 91 (1996): 187-91. [PMID: 8795442] | ||||
| 24 | Product Information. Actonel (risedronate). Procter and Gamble Pharmaceuticals, Cincinnati, OH. | ||||
| 25 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 26 | Bentley ML, Corwin HL, Dasta J "Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies." Crit Care Med 38(6 Suppl) (2010): S169-74. [PMID: 20502171] | ||||
| 27 | Bannister SJ, Houser VP, Hulse JD, Kisicki JC, Rasmussen JG "Evaluation of the potential for interactions of paroxetine with diazepam, cimetidine, warfarin, and digoxin." Acta Psychiatr Scand Suppl 350 (1989): 102-6. [PMID: 2530759] | ||||
| 28 | Ponce SP, Jennings AE, Madias NE, Harrington JT "Drug-induced hyperkalemia." Medicine (Baltimore) 64 (1985): 357-70. [PMID: 2865667] | ||||
| 29 | Product Information. Lovenox (enoxaparin). Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 30 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 31 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 32 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 33 | EMEA "EMEA public statement on leflunomide (ARAVA) - severe and serious hepatic reactions.". | ||||
| 34 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 35 | Muller FO, Schall R, Devaal AC, Groenewoud G, Hundt HKL, Middle MV "Influence of meloxicam on furosemide pharmacokinetics and pharmacodynamics in healthy volunteers." Eur J Clin Pharmacol 48 (1995): 247-51. [PMID: 7589049] | ||||
| 36 | Product Information. Canasa (mesalamine (5-aminosalicylic acid)). Axcan Scandipharm Inc, Birmingham, AL. | ||||
| 37 | Caruso V, Iacoviello L, Di Castelnuovo A, et.al "Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients." Blood 108 (2006): 2216-22. [PMID: 16804111] | ||||
| 38 | Product Information. Brukinsa (zanubrutinib). BeiGene USA, Inc, San Mateo, CA. | ||||
| 39 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 40 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 41 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 42 | Product Information. Zontivity (vorapaxar). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 43 | Abdel-Rahman MS, Reddi AS, Curro FA, Turkall RM, Kadry AM, Hansrote JA "Bioavailability of aspirin and salicylamide following oral co-administration in human volunteers." Can J Physiol Pharmacol 69 (1991): 1436-42. [PMID: 1777842] | ||||
| 44 | Product Information. Flolan (epoprostenol). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 45 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 46 | Bodiford AB, Kessler FO, Fermo JD, Ragucci KR "Elevated international normalized ratio with the consumption of grapefruit and use of warfarin." SAGE Open Med Case Rep 0 (2013): 1-3. [PMID: 27489634] | ||||
