Details of the Drug
General Information of Drug (ID: DM4QTBN)
| Drug Name |
Isotretinoin
|
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Accutane; Amnesteem; Claravis; Isotane; Isotretinoine; Isotretinoino; Isotretinoinum; Isotrex; Roaccutan; Roaccutane; Roacutan; Sotret; Teriosal; Neovitamin A acid; R 3255; Ro 4 3780; Ro 43780; Accutane (TN); Amnesteem (TN); BML2-E07; CIP-Isotretinoin; Claravi (TN); Clarus (TN); Decutan (TN); Isotane (TN); Isotretinoin (USP); Isotretinoin Zinc Salt, 13 cis Isomer; Isotretinoine [INN-French]; Isotretinoino [INN-Spanish]; Isotretinoinum [INN-Latin]; Izotek (TN); Oratane (TN); Ro 4-3780; Ro-43780; Roaccutane (TN); Sotret (TN); Isotretinoin [USAN:INN:BAN]; Ro-4-3780; Isotretinoin Zinc Salt, 13-cis-Isomer; (2Z,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid; (2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoic acid; (7E,9E,11E,13Z)-retinoic acid; 13-RA; 13-cis RA; 13-cis-Vitamin A acid; 3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)2-cis-4-trans-6-trans-8-trans-nonatetraenoic acid
|
||||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiacne Agents
|
||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||||
| Structure |
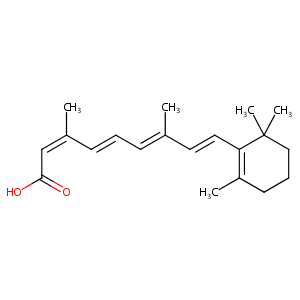 |
||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 300.4 | |||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.3 | ||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Isotretinoin
Coadministration of a Drug Treating the Disease Different from Isotretinoin (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7600). | ||||
|---|---|---|---|---|---|
| 2 | Isotretinoin FDA Label | ||||
| 3 | ClinicalTrials.gov (NCT04361422) Isotretinoin in Treatment of COVID-19. U.S. National Institutes of Health. | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 7 | Retinoid agonist isotretinoin ameliorates obstructive renal injury. J Urol. 2003 Oct;170(4 Pt 1):1398-402. | ||||
| 8 | The effect of isotretinoin on the pharmacokinetics and pharmacodynamics of ethinyl estradiol and norethindrone. Clin Pharmacol Ther. 2004 May;75(5):464-75. | ||||
| 9 | Temporal changes in gene expression in the skin of patients treated with isotretinoin provide insight into its mechanism of action. Dermatoendocrinol. 2009 May;1(3):177-87. | ||||
| 10 | Retinoic acid and its 4-oxo metabolites are functionally active in human skin cells in vitro. J Invest Dermatol. 2005 Jul;125(1):143-53. | ||||
| 11 | Gardner K, Cox T, Digre KB "Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review." Neurology 45 (1995): 6-10. [PMID: 7824136] | ||||
| 12 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 13 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 14 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 15 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 16 | Product Information. Piqray (alpelisib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 17 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
| 18 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 19 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 20 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 21 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 22 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 23 | Canadian Pharmacists Association. | ||||
| 24 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 25 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 26 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 27 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 28 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 29 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 30 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 31 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 32 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
