Details of the Drug
General Information of Drug (ID: DMDZ9LT)
| Drug Name |
Ethosuximide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aethosuccimidum; Aethosuximide; Asamid; Atysmal; Capitus; Emeside; Ethosuccimid; Ethosuccimide; Ethosuccinimide; Ethosuxide; Ethosuximidum; Ethylmethylsuccimide; Ethymal; Etomal; Etosuccimide; Etosuximid; Etosuximida; Etosuximide; Mesentol; Pemal; Pemalin; Pentinimid; Peptinimid; Petinimid; Petnidan; Piknolepsin; Pyknolepsinum; Ronton; Simatin; Succimal; Succimitin; Suksilep; Suxilep; Suximal; Suxin; Suxinutin; Thetamid; Thilopemal; Zaraondan; Zarodan; Zarondan; Zarontin; Zartalin; Aethosuximide [German]; Desitin Brand of Ethosuximide; Epileo Petit MAL; Etosuccimide [DCIT]; Etosuximida Faes; Faes Brand of Ethosuximide; Fortbenton Brand of Ethosuximide; Jenapharm Brand of Ethosuximide; Katwijk Brand of Ethosuximide; LAB Brand of Ethosuximide; Parke Davis Brand of Ethosuximide; Pfizer Brand of Ethosuximide; United Drug Brand of Ethosuximide; Warner Lambert Brand of Ethosuximide; Wernigerode Brand of Ethosuximide; Cl 366; E 7138; E0746; H 940; PM 671; CN-10395; Ethosuximidum [INN-Latin]; Etosuximida [INN-Spanish]; Faes, Etosuximida; H-490; N-Ethyl methylsuccinimide; PM-671; Simatin(E); Warner-Lambert Brand of Ethosuximide; Zarondan-Saft; Zarontin (TN); C.I. 366; CN-10,395; Piknole.psi.n; Pyknole.psi.num; Alpha-Ethyl-alpha-methylsuccinimide; Alpha-Methyl-alpha-ethylsuccinimide; Ethosuximide (JP15/USP/INN); Ethosuximide [USAN:INN:BAN:JAN]; Gamma-Methyl-gamma-ethylsuccinimide; Gamma-Methyl-gamma-ethyl-succinimide; Gamma-ethyl-gamma-methyl-succinimide; (+-)-2-Ethyl-2-methylsuccinimide; 2-Ethyl-2-methylsuccinimide; 2-Methyl-2-ethylsuccinimide; 3-Ethyl-3-methyl-2, 5-pyrrolidinedion; 3-Ethyl-3-methyl-2,5-pyrrolidinedione; 3-Ethyl-3-methylpyrrolidine-2,5-dione; 3-Ethyl-3-methylpyrroline-2,5-dione; 3-Ethyl-3-methylsuccinimide; 3-Methyl-3-ethylpyrrolidine-2,5-dione; 3-Methyl-3-ethylsuccinimide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
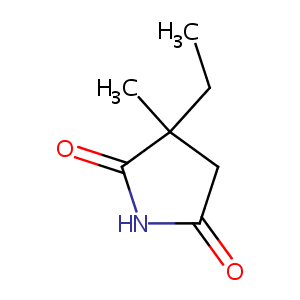 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 141.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Ethosuximide
Coadministration of a Drug Treating the Disease Different from Ethosuximide (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7182). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci. 2008 Nov 12;28(46):11959-69. | ||||
| 6 | Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of ethosuximide by human hepatic microsomal enzymes. Xenobiotica. 2003 Mar;33(3):265-76. | ||||
| 7 | Inhibition of human aromatase complex (CYP19) by antiepileptic drugs. Toxicol In Vitro. 2008 Feb;22(1):146-53. | ||||
| 8 | Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3. | ||||
| 9 | Product Information. Trileptal (oxcarbazepine) Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 10 | Product Information. Xcopri (cenobamate). SK Life Science, Inc., Paramus, NJ. | ||||
| 11 | Browne TR, Feldman RG, Buchanan RA, et al. "Methsuximide for complex partial seizures: efficacy, toxicity, clinical pharmacology, and drug interactions." Neurology 33 (1983): 414-8. [PMID: 6403891] | ||||
| 12 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 13 | Giaccone M, Bartoli A, Gatti G, Marchiselli R, Pisani F, Latella MA, Perucca E "Effect of enzyme inducing anticonvulsants on ethosuximide pharmacokinetics in epileptic patients." Br J Clin Pharmacol 41 (1996): 575-9. [PMID: 8799524] | ||||
| 14 | Product Information. Multaq (dronedarone). sanofi-aventis , Bridgewater, NJ. | ||||
| 15 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 16 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 17 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 18 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 19 | Belcastro V, Costa C, Striano P "Levetiracetam-associated hyponatremia." Seizure 17 (2008): 389-90. [PMID: 18584781] | ||||
| 20 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 21 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 22 | Product Information. Kalydeco (ivacaftor). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 23 | Product Information. Prevymis (letermovir). Merck & Company Inc, Whitehouse Station, NJ. | ||||
| 24 | Product Information. Emend (aprepitant). Merck & Company Inc, West Point, PA. | ||||
| 25 | Product Information. Tazverik (tazemetostat). Epizyme, Inc, Cambridge, MA. | ||||
| 26 | Auclair B, Berning SE, Huitt GA, Peloquin CP "Potential interaction between itraconazole and clarithromycin." Pharmacotherapy 19 (1999): 1439-44. [PMID: 10600094] | ||||
| 27 | Product Information. Victrelis (boceprevir). Schering-Plough Corporation, Kenilworth, NJ. | ||||
| 28 | Product Information. Incivek (telaprevir). Vertex Pharmaceuticals, Cambridge, MA. | ||||
| 29 | Product Information. Priftin (rifapentine). Hoechst Marion-Roussel Inc, Kansas City, MO. | ||||
| 30 | Product Information. Agenerase (amprenavir). Glaxo Wellcome, Research Triangle Pk, NC. | ||||
| 31 | Product Information. Stribild (cobicistat/elvitegravir/emtricitabine/tenofov). Gilead Sciences, Foster City, CA. | ||||
| 32 | Product Information. Sustiva (efavirenz). DuPont Pharmaceuticals, Wilmington, DE. | ||||
| 33 | Product Information. Fortovase (saquinavir) Roche Laboratories, Nutley, NJ. | ||||
| 34 | Product Information. Vaprisol (conivaptan). Cumberland Pharmaceuticals Inc, Nashville, TN. | ||||
| 35 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 36 | Product Information. Alunbrig (brigatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 37 | Product Information. Lorbrena (lorlatinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 38 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
| 39 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 40 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 41 | Product Information. Exjade (deferasirox). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 42 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 43 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 44 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 45 | Product Information. Gleevec (imatinib mesylate). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 46 | Doherty MM, Charman WN "The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism?" Clin Pharmacokinet 41 (2002): 235-53. [PMID: 11978143] | ||||
| 47 | Product Information. Alphagan (brimonidine ophthalmic). Allergan Inc, Irvine, CA. | ||||
| 48 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 49 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 50 | Sekar M, Mimpriss TJ "Buprenorphine, benzodiazepines and prolonged respiratory depression." Anaesthesia 42 (1987): 567-8. [PMID: 3592200] | ||||
| 51 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 52 | Product Information. Xenleta (lefamulin). Nabriva Therapeutics US, Inc., King of Prussia, PA. | ||||
| 53 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 54 | Benoist G, van Oort I, et al "Drug-drug interaction potential in men treated with enzalutamide: Mind the gap." Br J Clin Pharmacol 0 (2017): epub. [PMID: 28881501] | ||||
| 55 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 56 | Product Information. Oxbryta (voxelotor). Global Blood Therapeutics, Inc., South San Francisco, CA. | ||||
| 57 | DSouza DL, Levasseur LM, Nezamis J, Robbins DK, Simms L, Koch KM "Effect of alosetron on the pharmacokinetics of alprazolam." J Clin Pharmacol 41 (2001): 452-4. [PMID: 11304902] | ||||
| 58 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
| 59 | Product Information. Zanaflex (tizanidine). Acorda Therapeutics, Hawthorne, NY. | ||||
