Details of the Drug
General Information of Drug (ID: DM6RZ9Q)
| Drug Name |
Clonidine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
clonidine; Clonidin; Duraclon; Chlornidinum; 4205-90-7; Catapres-TTS; Catarpresan; Catarpres; Adesipress; Catapres; Catapressan; Catapresan; ST 155BS; ST-155-BS; Clonidinum; Clonidina; Clonidinum [INN-Latin]; Clonidinhydrochlorid; CATAPRES-TTS-3; CATAPRES-TTS-1; CATAPRES-TTS-2; N-(2,6-Dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine; Isoglaucon; 2-(2,6-Dichloroanilino)-2-imidazoline; Dixarit; SKF 34427; Catapres- TTS; Clonidina [INN-Spanish]; M-5041T; Hemiton; CLORPRES; clonidine (amino form); Adesipress; Clofenil; Clopheline; Duraclont; Gemiton; Klofelin; Klofenil; M 5041T; Catapres (TN); Catarpres-TTS; ST-155BS; Tenso-Timelets; Catarpres-TTS (TN); Clonidine [USAN:BAN:INN]; Clonidine (JAN/USAN/INN); 1H-Imidazol-2-amine, N-(2,6-dichlorophenyl)-4,5-dihydro-(9CI); 2,6-Dichloro-N-2-imidazolidinylidenebenzenamine; 2,6-dichloro-N-(2-imidazolidinylidene)aniline; 2,6-dichloro-N-2-imidazolidinylidenebenzenamide; 2,6-dichloro-N-imidazolidin-2-ylideneaniline; 2-((2,6-Dichlorophenyl)imino)imidazolidine; 2-(2,6-Dichloroanilino)-1,3-diazacyclopentene-(2);2-(2,6-Dichloroanilino)-2-imidazoline; 2-(2,6-Dichlorophenylamino)-2-imidazoline; 2-(2,6-Dichlorophenylimino)imidazolidine; 2-Imidazoline, 2-(2,6-dichloroanilino)-(7CI,8CI); 2-[(2,6-Dichlorophenyl)imino]imidazoline; 2-[(2,6-dichlorophenyl)imino]-2-imidazoline; 734571A; 2,6-DICHLORO-N-IMIDAZOLIDIN-2-YLIDENEANILINE
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
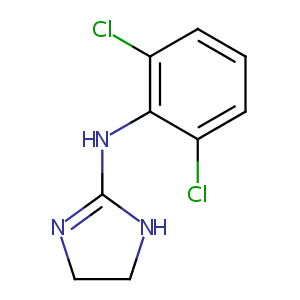 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 230.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Attention deficit hyperactivity disorder | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A05.Z | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Clonidine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Clonidine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 516). | ||||
| 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 4 | FDA Approved Drug Products: Catapres Clonidine Hydrochloride Oral Tablets | ||||
| 5 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 6 | alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999 Feb;54(2):146-65. doi: 10.1046/j.1365-2044.1999.00659.x. | ||||
| 7 | Chandrasekharan S: Pharmacokinetics of Dietary Isoflavones Journal of Steroids & Hormonal Science. | ||||
| 8 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 9 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 10 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 11 | Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharmacol Biochem Behav. 2000 Nov;67(3):397-403. | ||||
| 12 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | ||||
| 13 | Influx Transport of Cationic Drug at the Blood-Retinal Barrier: Impact on the Retinal Delivery of Neuroprotectants. Biol Pharm Bull. 2017;40(8):1139-1145. | ||||
| 14 | Caco-2 permeability, P-glycoprotein transport ratios and brain penetration of heterocyclic drugs. Int J Pharm. 2003 Sep 16;263(1-2):113-22. | ||||
| 15 | CYP2D6 mediates 4-hydroxylation of clonidine in vitro: implication for pregnancy-induced changes in clonidine clearance. Drug Metab Dispos. 2010 Sep;38(9):1393-6. | ||||
| 16 | The effects of clonidine hydrochloride versus atenolol monotherapy on serum lipids, lipid subfractions, and apolipoproteins in mild hypertension. Am Heart J. 1990 Jul;120(1):172-9. doi: 10.1016/0002-8703(90)90175-w. | ||||
| 17 | Clonidine Induces Apoptosis of Human Corneal Epithelial Cells through Death Receptors-Mediated, Mitochondria-Dependent Signaling Pathway. Toxicol Sci. 2017 Mar 1;156(1):252-260. doi: 10.1093/toxsci/kfw249. | ||||
| 18 | Ban TA "Drug interactions with psychoactive drugs." Dis Nerv Syst 36 (1975): 164-6. [PMID: 1116424] | ||||
| 19 | Aronowitz JS, Chakos MH, Safferman AZ, Lieberman JA "Syncope associated with the combination of clozapine and enalapril." J Clin Psychopharmacol 14 (1994): 429-30. [PMID: 7884028] | ||||
| 20 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 21 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 22 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 23 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 24 | Product Information. Thalomid (thalidomide). Celgene Corporation, Warren, NJ. | ||||
| 25 | Hansen BS, Dam M, Brandt J, et al "Influence of dextropropoxyphene on steady state serum levels and protein binding of three anti-epileptic drugs in man." Acta Neurol Scand 61 (1980): 357-67. [PMID: 6998251] | ||||
| 26 | Product Information. Clozaril (clozapine). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
